Key Concepts, Contexts & Terms
Academic Supervisor
REGULATIONS FOR THE PHILOSOPHIAE DOCTOR DEGREE (PHD) AT THE NORWEGIAN UNIVERSITY OF SCIENCE AND TECHNOLOGY (NTNU)
"Section 7.1 Appointment of academic supervisors
The Faculty appoints academic supervisors. As a general rule, the PhD candidate is to have at least two academic supervisors, of which one will be designated as the main supervisor. The main supervisor must be appointed at the time the candidate is admitted.
The main supervisor has the primary academic responsibility for the candidate. If the Faculty appoints an external main supervisor, a co-supervisor who is an academic staff member at NTNU is to be appointed.
Co-supervisors are experts in the field who provide academic supervision and who share the academic responsibility for the candidate with the main supervisor.
The provisions on impartiality in the Public Administration Act Chapter II concerning disqualification (Sections 6 to 10) [provided below] apply to the academic supervisors and appointed mentors.
All academic supervisors must hold a doctoral degree or equivalent qualification in the relevant research field and must be working actively as researchers. At least one of the appointed supervisors must have previous experience or training in academic supervision of PhD candidates.
In addition, the Faculty may appoint one or more mentors who do not meet the qualification requirements for supervisors, but who still provide supervisory assistance.
The PhD candidate and academic supervisor may ask the Faculty to appoint another supervisor for the candidate. The supervisor may not withdraw before a new supervisor has been appointed. Any disputes regarding the academic rights and obligations of the supervisor and of the candidate are to be referred by these parties to the Faculty for review and a final decision.
Section 7.2 Content of the academic supervision
The supervisors are to give advice on formulating and delimiting the thematic area and research questions, discuss and assess hypotheses and methods, discuss the results and the interpretation of these, discuss the structure and work on the thesis, including the outline, choice of language, documentation, etc., and provide guidance on the academic literature and data available in libraries, archives, etc. The supervisors must also advise the candidate on issues related to research ethics in connection with the thesis.
The candidate must have regular contact with his or her supervisors. The frequency of contact between the parties is to be stated in the annual reporting of progress; cf. Section 9.
The candidate and supervisors have a mutual obligation to keep each other informed about the PhD Regulations for NTNU Approved by the Board of NTNU on 23 January 2012 7 progress of the work and to assess it in relation to the project description.
The supervisors are required to follow up academic issues that may cause a delay in the progression of the candidate’s PhD education, so that it can be completed within the nominal period of study."
(Source: PhD Regulations for NTNU Approved by the Board of NTNU on 23 January 2012: REGULATIONS FOR THE PHILOSOPHIAE DOCTOR DEGREE (PHD) AT THE NORWEGIAN UNIVERSITY OF SCIENCE AND TECHNOLOGY (NTNU) Norwegian: Forskrift for graden philosophiae doctor (ph.d.) ved Norges teknisk-naturvitenskapelig universitet (NTNU))
Act relating to procedure in cases concerning the public administration (Public Administration Act)
"Chapter II. Concerning disqualification
Section 6. (requirements as to impartiality)
§ 6. (habilitetskrav).
A public official shall be disqualified from preparing the basis for a decision or from making any decision in an administrative case
If the superior official is disqualified, the case may not be decided by any directly subordinate official in the same administrative agency.
The rules governing disqualification shall not apply if it is evident that the official's connection with the case or the parties will not influence his standpoint and neither public nor private interests indicate that he should stand down.
The scope of the second and fourth paragraphs may be further specified in regulations prescribed by the King."
Section 7. (Provisional decision) Regardless of whether an official is disqualified, he may deal with a case or make a provisional decision in a case if it cannot be postponed without causing considerable inconvenience or harm.
Section 8. (Decision concerning the question of disqualification)
The official shall himself decide whether he is disqualified. He shall submit the question to his immediate superior for decision if a party so requests and this may be done without undue loss of time, or if the official himself otherwise finds reason to do so.
In collegiate bodies the decision shall be made by the body itself, without the participation of the member concerned. If, in one and the same case, the question of disqualification should arise in respect of several members, none of them may participate in the decision regarding their own or another member's disqualification, unless the collegiate body would otherwise lack a quorum for deciding the question. In the latter case all attending members shall participate.
A member shall give ample notice of any circumstance which disqualifies or may disqualify him. Before the question is decided, his deputy or other substitute should be summoned to attend and participate in the decision if this may be done without undue expense or loss of time.
Section 9. (Appointment of a substitute)
If an official is disqualified, a substitute shall, if necessary, be appointed or elected in his stead.
If the appointment of a substitute will be particularly inconvenient, the King may decide that the case in question shall be transferred to a coordinate or superior administrative agency
Section 10. (Persons to whom the rules on disqualification shall apply) Besides public officials, the provisions of this Chapter shall apply correspondingly to any other person who performs services or work for an administrative agency. The provisions shall not apply to members of the Council of State in their capacity as members of the government."
(Source: Lovdata.no: English: Act relating to procedure in cases concerning the public administration (Public Administration Act) Norwegian: Lov om behandlingsmåten i forvaltningssaker (forvaltningsloven) (Forvaltningsloven – fvl)
Authorship
Who Is an Author?
International Committee of Medical Journal Editors (ICMJE)
"The ICMJE recommends that authorship be based on the following 4 criteria:
Authorship (continued)
"Scientific integrity, truthfulness and accountability"
Authorship (continued)
"Misusing authorship to build career" ("Misbruker forfatterskap for å bygge karriere")
Authorship (continued)
"Freeloading in biomedical research"
"Thousands of scientists publish a paper every five days"
To highlight uncertain norms in authorship, John P. A. Ioannidis, Richard Klavans and Kevin W. Boyack identified the most prolific scientists of recent years.
Birth
Citation Distortions
"How citation distortions create unfounded authority: analysis of a citation network"
"Vocabulary of citation distortions
Citation
Citation Manipulation
"Citation manipulation refers to the following types of behaviour:
Citation Doping & Stacking
"Hundreds of extreme self-citing scientists revealed in new database"
"Some highly cited academics seem to be heavy self-promoters — but researchers warn against policing self-citation."
"Italy’s rise in research impact pinned on ‘citation doping'"
"Citation of Italian-authored papers by Italian researchers rose after the introduction of metrics-based thresholds for promotions."
"Brazilian citation scheme outed"
"Thomson Reuters suspends journals from its rankings for ‘citation stacking’."
Cognitive dissonance "In the field of psychology, cognitive dissonance is the mental discomfort (psychological stress) experienced by a person who simultaneously holds two or more contradictory beliefs, ideas, or values. The occurrence of cognitive dissonance is a consequence of a person's performing an action that contradicts personal beliefs, ideals, and values; and also occurs when confronted with new information that contradicts said beliefs, ideals, and values." (Source: Wikipedia: Cognitive dissonance)
Confirmation bias "the tendency to interpret new evidence as confirmation of one's existing beliefs or theories." (Source: Wikipedia: Confirmation bias)
Conflate-to-obfuscate a technique whereby two or more sets of information, texts, ideas, methods, etc. are combined into one set and presented to be synonymous in an effort to confuse the real differences between or among them. This is also known as combine-to-confuse and fuse-to-confuse. (See: "Confused on conflated" Merrill Perlman. Columbia Journalism Review February 9, 2015)
“The rise of Italy in impact rankings appears to be the fruit of a collective ‘citation doping’ induced by the new policies,” says Alberto Baccini, an economist at the University of Siena in Italy who led the study, published on 11 September in PLoS ONE1.
REGULATIONS FOR THE PHILOSOPHIAE DOCTOR DEGREE (PHD) AT THE NORWEGIAN UNIVERSITY OF SCIENCE AND TECHNOLOGY (NTNU)
"Section 7.1 Appointment of academic supervisors
The Faculty appoints academic supervisors. As a general rule, the PhD candidate is to have at least two academic supervisors, of which one will be designated as the main supervisor. The main supervisor must be appointed at the time the candidate is admitted.
The main supervisor has the primary academic responsibility for the candidate. If the Faculty appoints an external main supervisor, a co-supervisor who is an academic staff member at NTNU is to be appointed.
Co-supervisors are experts in the field who provide academic supervision and who share the academic responsibility for the candidate with the main supervisor.
The provisions on impartiality in the Public Administration Act Chapter II concerning disqualification (Sections 6 to 10) [provided below] apply to the academic supervisors and appointed mentors.
All academic supervisors must hold a doctoral degree or equivalent qualification in the relevant research field and must be working actively as researchers. At least one of the appointed supervisors must have previous experience or training in academic supervision of PhD candidates.
In addition, the Faculty may appoint one or more mentors who do not meet the qualification requirements for supervisors, but who still provide supervisory assistance.
The PhD candidate and academic supervisor may ask the Faculty to appoint another supervisor for the candidate. The supervisor may not withdraw before a new supervisor has been appointed. Any disputes regarding the academic rights and obligations of the supervisor and of the candidate are to be referred by these parties to the Faculty for review and a final decision.
Section 7.2 Content of the academic supervision
The supervisors are to give advice on formulating and delimiting the thematic area and research questions, discuss and assess hypotheses and methods, discuss the results and the interpretation of these, discuss the structure and work on the thesis, including the outline, choice of language, documentation, etc., and provide guidance on the academic literature and data available in libraries, archives, etc. The supervisors must also advise the candidate on issues related to research ethics in connection with the thesis.
The candidate must have regular contact with his or her supervisors. The frequency of contact between the parties is to be stated in the annual reporting of progress; cf. Section 9.
The candidate and supervisors have a mutual obligation to keep each other informed about the PhD Regulations for NTNU Approved by the Board of NTNU on 23 January 2012 7 progress of the work and to assess it in relation to the project description.
The supervisors are required to follow up academic issues that may cause a delay in the progression of the candidate’s PhD education, so that it can be completed within the nominal period of study."
(Source: PhD Regulations for NTNU Approved by the Board of NTNU on 23 January 2012: REGULATIONS FOR THE PHILOSOPHIAE DOCTOR DEGREE (PHD) AT THE NORWEGIAN UNIVERSITY OF SCIENCE AND TECHNOLOGY (NTNU) Norwegian: Forskrift for graden philosophiae doctor (ph.d.) ved Norges teknisk-naturvitenskapelig universitet (NTNU))
Act relating to procedure in cases concerning the public administration (Public Administration Act)
"Chapter II. Concerning disqualification
Section 6. (requirements as to impartiality)
§ 6. (habilitetskrav).
A public official shall be disqualified from preparing the basis for a decision or from making any decision in an administrative case
- a) if he himself is a party to the case;
- b) if he is related by blood or by marriage to a party in direct line of ascent or descent, or collaterally as close as a sibling;
- c) if he is or has been married or is engaged to a party, or is the foster parent or foster child of a party;
- d) if he is the guardian or agent of a party to the case or has been the guardian or agent of a party after the case began;
- e) if he is the head of, or holds a senior position in, or is a member of the board of directors or the corporate assembly of
- 1.a cooperative company, or an association, savings bank or foundation that is a party to the case, or
- 2.a company which is a party to the case. Nevertheless, this does not apply to a person who performs services or work for a company that is wholly-owned by the State and/or a municipality, and such company, either alone or together with other similar companies or the State and/or a municipality, wholly owns the company that is a party to the case.
If the superior official is disqualified, the case may not be decided by any directly subordinate official in the same administrative agency.
The rules governing disqualification shall not apply if it is evident that the official's connection with the case or the parties will not influence his standpoint and neither public nor private interests indicate that he should stand down.
The scope of the second and fourth paragraphs may be further specified in regulations prescribed by the King."
Section 7. (Provisional decision) Regardless of whether an official is disqualified, he may deal with a case or make a provisional decision in a case if it cannot be postponed without causing considerable inconvenience or harm.
Section 8. (Decision concerning the question of disqualification)
The official shall himself decide whether he is disqualified. He shall submit the question to his immediate superior for decision if a party so requests and this may be done without undue loss of time, or if the official himself otherwise finds reason to do so.
In collegiate bodies the decision shall be made by the body itself, without the participation of the member concerned. If, in one and the same case, the question of disqualification should arise in respect of several members, none of them may participate in the decision regarding their own or another member's disqualification, unless the collegiate body would otherwise lack a quorum for deciding the question. In the latter case all attending members shall participate.
A member shall give ample notice of any circumstance which disqualifies or may disqualify him. Before the question is decided, his deputy or other substitute should be summoned to attend and participate in the decision if this may be done without undue expense or loss of time.
Section 9. (Appointment of a substitute)
If an official is disqualified, a substitute shall, if necessary, be appointed or elected in his stead.
If the appointment of a substitute will be particularly inconvenient, the King may decide that the case in question shall be transferred to a coordinate or superior administrative agency
Section 10. (Persons to whom the rules on disqualification shall apply) Besides public officials, the provisions of this Chapter shall apply correspondingly to any other person who performs services or work for an administrative agency. The provisions shall not apply to members of the Council of State in their capacity as members of the government."
(Source: Lovdata.no: English: Act relating to procedure in cases concerning the public administration (Public Administration Act) Norwegian: Lov om behandlingsmåten i forvaltningssaker (forvaltningsloven) (Forvaltningsloven – fvl)
Authorship
Who Is an Author?
International Committee of Medical Journal Editors (ICMJE)
"The ICMJE recommends that authorship be based on the following 4 criteria:
- Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
- Drafting the work or revising it critically for important intellectual content; AND
- Final approval of the version to be published; AND
- Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved." (Source: International Committee of Medical Journal Editors, "Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals Updated December 2016" http://www.icmje.org/ & http://www.icmje.org/icmje-recommendations.pdf, resectively)
Authorship (continued)
"Scientific integrity, truthfulness and accountability"
- "5 Researchers must respect the contributions of other researchers and observe standards of authorship and cooperation.
Researchers must observe good publication practice. They must clarify individual responsibilities in group work as well as the rules for co-authorship. Honorary authorship is unacceptable. When several authors contribute, each authorship must be justified. Justified authorship is defined by four criteria, in accordance with the criteria drawn up by the International Committee of Medical Journal Editors (ICMJE) 3:
a) Researchers must have made a substantial contribution to the conception and design or the data acquisition or the data analysis and interpretation; and
b) researchers must have contributed to drafting the manuscript or critical revision of the intellectual content of the publication; and
c) researchers must have approved the final version before publication; and
d) researchers must be able to accept responsibility for and be accountable for the work as a whole (albeit not necessarily all technical details) unless otherwise specified.
All authors in a multidisciplinary publication must be able to account for the part or parts for which they have been responsible in the research work, and which part or parts are the responsibility of other contributors.
All those who meet criterion a) must be able to meet b) and c). Contributors who do not fulfil all the criteria must be acknowledged." (Source: "Scientific integrity, truthfulness and accountability" from "Guidelines for research ethics in science and technology" Issued by The Norwegian National Committee for Research Ethics in Science and Technology (2016). Text: The Norwegian National Committees for Research Ethics, Last updated: Tuesday, June 28, 2016)
Authorship (continued)
"Misusing authorship to build career" ("Misbruker forfatterskap for å bygge karriere")
- Authorship is easy to count, but difficult to handle. Every third researcher admits having awarded co-authorship to one who does not deserve it.
Forfatterskap er lett å telle, men vanskelig å håndtere. Hver tredje forsker innrømmer å ha tildelt medforfatterskap til en som ikke fortjener det. (Source: "Misusing authorship to build career" ("Misbruker forfatterskap for å bygge karriere") Ida Irene Bergstrøm. De nasjonale forskningsetiske komiteene. Sist oppdatert: 21. mars 2018. Original version: "Honor Where Honor is Due" ("Æres den som æres bør") Ida Irene Bergstrøm, Editor/Redaktor Forsknings etikk Et magasin fra De nasjonale forskningsetiske komiteene. NR. 1 • Mars 2018 • 18. årgang. p. 4-7.) - When the International Committee for Medical Journal Editors (ICMJE) made their first guidelines for articles in biomedical journals in 1979, no authorship was mentioned, says Charlotte Haug.
Haug is an international correspondent for The New England Journal of Medicine, and senior researcher at SINTEF. From 2002-2015 she was editor of the magazine for the Norwegian Medical Association.
Da den internasjonale komiteen for medisinske tidsskriftredaktører (ICMJE) lagde sine første retningslinjer for artikler i biomedisinske tidsskrifter i 1979, var ikke forfatterskap nevnt, forteller Charlotte Haug.
Haug er internasjonal korrespondent for The New England Journal of Medicine, og seniorforsker ved SINTEF. Fra 2002-2015 var hun redaktør for Tidsskrift for den norske legeforening.
"In 1979, it was thought that everyone had the same meaning about what the authorship meant," said Haug.
Only in 1988 came the first criteria for authorship in the ICMJE Guidelines, also known as the Vancouver Recommendations."
– I 1979 tenkte man at alle hadde samme mening om hva forfatterskap innebar, sier Haug.
Først i 1988 kom de første kriteriene om forfatterskap i ICMJE-retningslinjene, også kjent som Vancouveranbefalingene.
"The following updates have been about refining these first criteria," says Haug.
– Påfølgende oppdateringer har handlet om å finpusse disse første kriteriene, forteller Haug.
"Until 2000, it was argued whether those who had gathered data and contributed in analyzes should be included. Over the 2000s, a number of profiled cases came about falsification and manufacturing.
In 2013, therefore, we received a fourth criterion that states that everyone is responsible for the entire article. That means everyone is responsible for saying that "I believe in this - and I think something is wrong, I am responsible for investigating it."
– Frem til 2000 kranglet man om hvorvidt de som hadde samlet inn data og bidratt i analyser skulle få være med. Utover 2000-tallet kom en rekke profilerte saker om falsifisering og fabrikkering.
I 2013 fikk vi derfor et fjerde kriterium som sier at alle er ansvarlige for hele artikkelen. Det betyr at alle er ansvarlige for å si at «jeg tror på dette – og tror jeg noe er feil, har jeg ansvar for å få det undersøkt».
Other discussions have been about wrongful authors who have not contributed and thus should not have their name there. This is often called honorary authorship or guest authorship. Another problem is ghost writers - writers who have contributed or might be behind the entire article, but do not stand as authors.
Andre diskusjoner har handlet om urettmessige forfattere som ikke har bidratt og dermed ikke burde hatt sitt navn der. Dette kalles gjerne æresforfatterskap, eller gjesteforfatterskap. Et annet problem er ghost writers – forfattere som har bidratt eller kanskje står bak hele artikkelen, men ikke står som forfattere.
"Several cases revealed that agencies had been paid by the pharmaceutical industry to write articles," says Haug.
– Flere saker avslørte at byråer hadde blitt betalt av legemiddelindustrien for å skrive artikler, forteller Haug. (Source: ibid.) - Charlotte Haug recalls that the ICMJE criteria have been developed by editors, to know who they can hold responsible for the text they publish.
Charlotte Haug minner om at ICMJE-kriteriene er utviklet av redaktører, for å vite hvem de kan holde ansvarlig for teksten de publiserer.- "There is a reason why this type of criteria was first developed in medicine and health. It is essential that research teams have done what has been done. Anything else can actually put patients at risk. The ICMJE guidelines are not just four criteria," emphasizes the former journal editor. "There are lots of text that elaborate on what they are about and how they should be interpreted."
– Det er en grunn til at denne typen kriterier først ble utviklet i medisin og helse. Det er helt vesentlig at det er hold i forskningen, at man har gjort det som står der. Noe annet kan faktisk sette pasienter i fare.
ICMJE-retningslinjene er dessuten ikke bare fire kriterier, understreker den tidligere tidsskriftredaktøren. Det er masse tekst som utdyper hva de handler om og hvordan de skal tolkes.
"There is an incredible amount of conflict tied to authorship, it is simply an incredibly large conflict area. It's hardly possible to believe if you have not been in the research world, "says Haug.
– Det er utrolig mye konflikt knytta til forfatterskap, det er rett og slett et utrolig stort konfliktområde. Det er nesten ikke til å tro hvis du ikke har vært inne i forskningsverdenen, sier Haug.
"At the same time, multi-literacy in medicine is a form of quality assurance. You should not sit in a room alone and keep up with yours. It's not that you necessarily cheat consciously, but you can leave results that do not fit completely, slip away, or move some numbers. It is easy and human to believe very much in one's own hypothesis. Therefore, you must be more. If Sudbø had been the sole author, as it practically looked like he was, the editors would have suspected at once."
– Samtidig er det sånn at flerforfatterskap innen medisinen er en form for kvalitetssikring. Du skal ikke sitte i et rom alene og holde på med ditt. Det er ikke det at man da nødvendigvis jukser bevisst, men man kan la resultater som ikke passer helt, gli bort, eller flytte noen tall. Det er lett og menneskelig å tro veldig på sin egen hypotese. Derfor skal man være flere. Hvis Sudbø hadde vært eneforfatter, som det i praksis nesten så ut som han var, så ville redaktørene fattet mistanke med en gang. (Source: ibid.)
- "There is a reason why this type of criteria was first developed in medicine and health. It is essential that research teams have done what has been done. Anything else can actually put patients at risk. The ICMJE guidelines are not just four criteria," emphasizes the former journal editor. "There are lots of text that elaborate on what they are about and how they should be interpreted."
- However, Charlotte Haug is tired of discussing authorship criteria.
Charlotte Haug er imidlertid lei av å diskutere forfatterskapskriterier.- "This should be expressed in discussions about what is happening at the institutions. What kind of culture it is for collaboration, for sharing data. It's a bit like #metoo. We can hold on for a while and take the bad ones. But first and foremost, this is about culture that is established over a long period of time, which makes it clear what is going beyond borders. It should trigger discussions about how we actually organize and conduct research. What we mean by researching and writing together."
– Det dette bør munne ut i er diskusjoner om hva som foregår på institusjonene. Hva slags kultur det er for samarbeid, for deling av data. Det er litt som med #metoo. Vi kan godt holde på en stund til og ta de slemme. Men først og fremst dreier dette seg om kultur som etableres over lang tid, der det blir klart hva som er å overskride grenser. Det bør utløse diskusjoner om hvordan vi egentlig organiserer og driver med forskning. Hva vi mener med å forske, og å skrive sammen. (Source: ibid.)
- "This should be expressed in discussions about what is happening at the institutions. What kind of culture it is for collaboration, for sharing data. It's a bit like #metoo. We can hold on for a while and take the bad ones. But first and foremost, this is about culture that is established over a long period of time, which makes it clear what is going beyond borders. It should trigger discussions about how we actually organize and conduct research. What we mean by researching and writing together."
Authorship (continued)
"Freeloading in biomedical research"
- Abstract
The surge in the number of authors per article in the biomedical field makes it difficult to quantify the contribution of individual authors. Conventional citation metrics are typically based on the number of publications and the number of citations generated by a scientist, thereby disregarding the contribution of co-authors. Previously we developed the p-index that estimates the dependency of a scientist on co-authors during their career. In this study we aimed to evaluate the ability of the p-index to identify researchers with a relatively high degree of scientific dependence on co-authors. For this purpose, we retrieved articles, which were rejected for publication in Journal of Thrombosis and Haemostasis and subsequently published elsewhere. Assuming that authors who were added to a later version of these articles would not fulfill the full authorship criteria, we tested whether these authors showed a larger dependency on co-authors during their scientific career as would be evident from a higher p-index. In accordance with this hypothesis, authors who were added on later versions of articles showed a higher p-index than their peers, indicating an enduring pattern of dependency on other co-authors for publishing their work. This study underscores that questionable authorship practices are endemic to the biomedical research, which calls for alternative methods to evaluate a scientist’s qualities. (Source: "Freeloading in biomedical research" Rozing, M.P., van Leeuwen, T.N., Reitsma, P.H. et al. Scientometrics (2019). https://doi.org/10.1007/s11192-018-2984-3)
"Thousands of scientists publish a paper every five days"
To highlight uncertain norms in authorship, John P. A. Ioannidis, Richard Klavans and Kevin W. Boyack identified the most prolific scientists of recent years.
- "Widely used citation and impact metrics should be adjusted accordingly. For instance, if adding more authors diminished the credit each author received, unwarranted multi-authorship might go down. We found that the 30 hyperprolific authors who seemed to benefit the most from co-authorship numbered 6 cardiologists and 24 epidemiologists (including those working on population genetics studies). (For these scientists, the ratio of their Hirsch H index to their co-authorship-adjusted Schreiber Hm index was higher; see Supplementary Information.)
Overall, hyperprolific authors might include some of the most energetic and excellent scientists. However, such modes of publishing might also reflect idiosyncratic field norms, to say the least. Loose definitions of authorship, and an unfortunate tendency to reduce assessments to counting papers, muddy how credit is assigned. One still needs to see the total publishing output of each scientist, benchmarked against norms for their field. And of course, there is no substitute for reading the papers and trying to understand what the authors have done." (Source: "Thousands of scientists publish a paper every five days" To highlight uncertain norms in authorship, John P. A. Ioannidis, Richard Klavans and Kevin W. Boyack identified the most prolific scientists of recent years. Nature 561, 167-169 (2018) 12 SEPTEMBER 2018. doi: 10.1038/d41586-018-06185-8)
Birth
- Post-term birth birth >=42 completed gestational weeks
- Preterm birth birth at a gestational age <37 completed gestational weeks
- Term birth birth >=37 and <42 completed gestational weeks
Citation Distortions
"How citation distortions create unfounded authority: analysis of a citation network"
"Vocabulary of citation distortions
Citation
- Both scholarly and social forms: the scholarly form connects statements to the broader medical literature, the social form (social citation) includes self serving and persuasive subtypes
- Self serving citation is always a distortion
- Persuasive citation may be necessary to communicate new, sound claims to the scientific community; it may, however, have distorted uses—citation bias, amplification, and invention
- Systematic ignoring of papers that contain content conflicting with a claim
- Bolster claim; justifying animal models to provide opportunities to amplify claim
- Expansion of a belief system without data
- Citation made to papers that don’t contain primary data, increasing the number of citations supporting the claim without presenting data addressing it
- Citation diversion—citing content but claiming it has a different meaning, thereby diverting its implication
- Citation transmutation—the conversion of hypothesis into fact through the act of citation alone
- Back door invention—repeated misrepresentation of abstracts as peer reviewed papers to fool readers into believing that claims are based on peer reviewed published methods and data
- Dead end citation—support of a claim with citation to papers that do not contain content addressing the claim
- Title invention—reporting of “experimental results” in a paper’s title, even though the paper does not report the performance or results of any such experiments"
(Source: "How citation distortions create unfounded authority: analysis of a citation network" Steven A Greenberg. BMJ 2009;339:b2680)
Citation Manipulation
"Citation manipulation refers to the following types of behaviour:
- Excessive citation of an author’s research by the author (ie, self-citation by authors) as a means solely of increasing the number of citations of the author’s work;
- Excessive citation of articles from the journal in which the author is publishing a research article as a means solely of increasing the number of citations of the journal; or
- Excessive citation of the work of another author or journal, sometimes referred to as ‘honorary’ citations (eg, the editor-in-chief of the journal to which one is submitting a manuscript or a well-known scholar in the field of the researcher) or ‘citation stacking’ solely to contribute to the citations of the author(s)/ journal(s) in question.
Citation Doping & Stacking
"Hundreds of extreme self-citing scientists revealed in new database"
"Some highly cited academics seem to be heavy self-promoters — but researchers warn against policing self-citation."
- "Vaidyanathan, a computer scientist at the Vel Tech R&D Institute of Technology, a privately run institute, is an extreme example: he has received 94% of his citations from himself or his co-authors up to 2017, according to a study in PLoS Biology this month1. He is not alone. The data set, which lists around 100,000 researchers, shows that at least 250 scientists have amassed more than 50% of their citations from themselves or their co-authors, while the median self-citation rate is 12.7%."
The study could help to flag potential extreme self-promoters, and possibly ‘citation farms’, in which clusters of scientists massively cite each other, say the researchers. “I think that self-citation farms are far more common than we believe,” says John Ioannidis, a physician at Stanford University in California who specializes in meta-science — the study of how science is done — and who led the work. “Those with greater than 25% self-citation are not necessarily engaging in unethical behaviour, but closer scrutiny may be needed,” he says." (Source: "Hundreds of extreme self-citing scientists revealed in new database" Richard Van Noorden & Dalmeet Singh Chawla, Nature 572, 578-579 (2019) doi: 10.1038/d41586-019-02479-7)
"Italy’s rise in research impact pinned on ‘citation doping'"
"Citation of Italian-authored papers by Italian researchers rose after the introduction of metrics-based thresholds for promotions."
- "Italy is climbing international rankings of research impact, but that doesn’t mean the country’s science has improved or become more influential. An analysis suggests the upward trend could be largely the result of Italian academics referencing each other’s articles more heavily to satisfy controversial management targets at universities.
The rise of Italy in impact rankings appears to be the fruit of a collective ‘citation doping’ induced by the new policies,” says Alberto Baccini, an economist at the University of Siena in Italy who led the study, published on 11 September in PLoS ONE." (Source: "Italy’s rise in research impact pinned on ‘citation doping’" Richard Van Noorden, Nature, 13 SEPTEMBER 2019, https://doi.org/10.1371/journal.pone.0221212 (2019))
"Brazilian citation scheme outed"
"Thomson Reuters suspends journals from its rankings for ‘citation stacking’."
- "Mauricio Rocha-e-Silva thought that he had spotted an easy way to raise the profiles of Brazilian journals. From 2009, he and several other editors published articles containing hundreds of references to papers in each others’ journals — in order, he says, to elevate the journals’ impact factors.
Because each article avoided citing papers published by its own journal, the agreement flew under the radar of analyses that spot extremes in self-citation — until 19 June, when the pattern was discovered. Thomson Reuters, the firm that calculates and publishes the impact factor, revealed that it had designed a program to spot concentrated bursts of citations from one journal to another, a practice that it has dubbed ‘citation stacking’. Four Brazilian journals were among 14 to have their impact factors suspended for a year for such stacking. And in July, Rocha-e-Silva was fired from his position as editor of one of them, the journal Clinics, based in São Paulo." - "The journals flagged by the new algorithm extend beyond Brazil — but only in that case has an explanation for the results emerged. Rocha-e-Silva says the agreement grew out of frustration with his country’s fixation on impact factor. In Brazil, an agency in the education ministry, called CAPES, evaluates graduate programmes in part by the impact factors of the journals in which students publish research. As emerging Brazilian journals are in the lowest ranks, few graduates want to publish in them. This vicious cycle, in his view, prevents local journals improving."
Cognitive dissonance "In the field of psychology, cognitive dissonance is the mental discomfort (psychological stress) experienced by a person who simultaneously holds two or more contradictory beliefs, ideas, or values. The occurrence of cognitive dissonance is a consequence of a person's performing an action that contradicts personal beliefs, ideals, and values; and also occurs when confronted with new information that contradicts said beliefs, ideals, and values." (Source: Wikipedia: Cognitive dissonance)
Confirmation bias "the tendency to interpret new evidence as confirmation of one's existing beliefs or theories." (Source: Wikipedia: Confirmation bias)
Conflate-to-obfuscate a technique whereby two or more sets of information, texts, ideas, methods, etc. are combined into one set and presented to be synonymous in an effort to confuse the real differences between or among them. This is also known as combine-to-confuse and fuse-to-confuse. (See: "Confused on conflated" Merrill Perlman. Columbia Journalism Review February 9, 2015)
- Conflate: combine (two or more sets of information, texts, ideas, etc.) into one
- Obfuscate: make obscure, unclear, or unintelligible; bewilder (someone)
“The rise of Italy in impact rankings appears to be the fruit of a collective ‘citation doping’ induced by the new policies,” says Alberto Baccini, an economist at the University of Siena in Italy who led the study, published on 11 September in PLoS ONE1.
Corruption
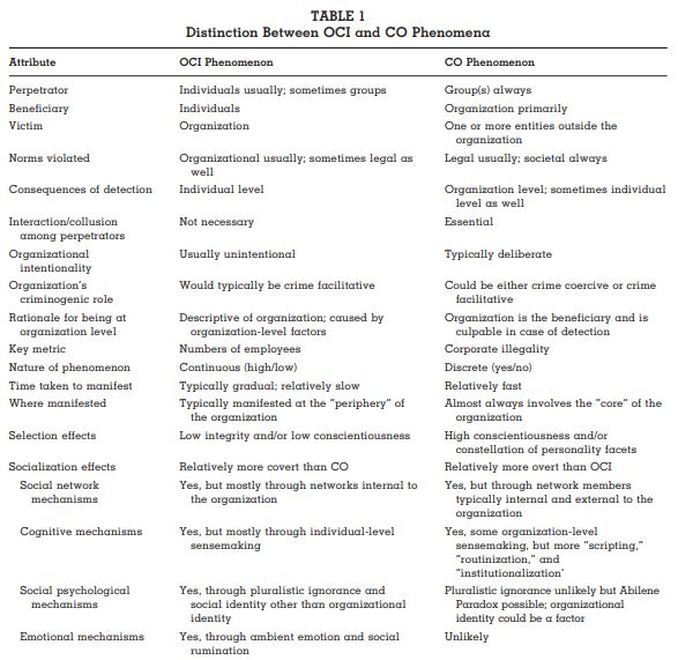
"CORRUPT ORGANIZATIONS OR ORGANIZATIONS OF CORRUPT INDIVIDUALS? TWO TYPES OF ORGANIZATION-LEVEL CORRUPTION"
"CORRUPT ORGANIZATIONS OR ORGANIZATIONS OF CORRUPT INDIVIDUALS? TWO TYPES OF ORGANIZATION-LEVEL CORRUPTION"
- "We focus on two fundamental dimensions of corruption in organizations: (1) whether the individual or the organization is the beneficiary of the corrupt activity and (2) whether the corrupt behavior is undertaken by an individual actor or by two or more actors. We use these dimensions to define a new conceptualization of corruption at the organization level: the organization of corrupt individuals. We contrast this conceptualization with the prevailing concept of organizational corruption and develop propositions that highlight their differences." (Source: "CORRUPT ORGANIZATIONS OR ORGANIZATIONS OF CORRUPT INDIVIDUALS? TWO TYPES OF ORGANIZATION-LEVEL CORRUPTION" JONATHAN PINTO, CARRIE R. LEANA, FRITS K. PIL. Academy of Management Review 2008, Vol. 33, No. 3, 685–709. p. 685)
- '"We offer our typology of corruption as a way of clarifying theory and research at the organization level. We also aim to address why different types of organizations may manifest different corruption phenomena, or why attempting to control one form of corruption may unwittingly lead to the creation of the other. Our multilevel approach encompasses bottom-up and top-down corruption, both composition and compilation emergent processes, and both selection and socialization in organizations. Finally, we address the relatively small but important literature on criminogenic mechanisms—that is, mechanisms that produce or tend to produce crime or criminal behavior. While this literature focuses almost exclusively on “crime-coercive” mechanisms, we focus more on “crime-facilitative” ones (Needleman & Needleman, 1979). (Source: Ibid., p. 685-686)
- "TWO TYPES OF CORRUPTION AT THE ORGANIZATION LEVEL
Using the two dimensions of beneficiary and collusion, we suggest that corruption at the organization level can manifest itself through two very distinct phenomena: an organization of corrupt individuals (OCI), in which a significant proportion of an organization’s members act in a corrupt manner primarily for their personal benefit (similar to the economics perspective), and a corrupt organization (CO), in which a group collectively acts in a corrupt manner for the benefit of the organization (similar to the sociology perspective). In both forms of corruption, the organization is the focal unit—that is, the level to which generalizations are made—and both the levels of measurement and the levels of analysis can be at the individual, group, organization, and environmental levels. (Source: Ibid., p 688)
Council of Science Editors is a dynamic community of editorial professionals dedicated to the responsible and effective communication of science.
Dates All dates are in dd.mm.yyyy format unless otherwise indicated.
Death
Dolichocephaly, more commonly "long head" or "breech head"; Cephalic index (CI) <= 75
Doublethink "Doublethink is the act of simultaneously accepting two mutually contradictory beliefs as correct, often in distinct social contexts. [1] Doublethink is related to, but differs from, hypocrisy and neutrality. Also related is cognitive dissonance, in which contradictory beliefs cause conflict in one's mind. Doublethink is notable due to a lack of cognitive dissonance—thus the person is completely unaware of any conflict or contradiction." (Source: Wikipedia: Doublethink)
Estimation vs. Prediction
The terms "estimation" and "prediction" have been conflated and and are commonly used interchangeably as synonyms in the ultrasound biometry literature, which is unfortunate. Consequently, this document will also use these terms somewhat interchangeably. However, in classical statistical inference there is a clear distinction between an estimator and predictor. An estimator uses data to guess at a parameter or coefficient while a predictor (e.g., an ultrasound fetal metric measurement) uses data to guess at some random value (e.g., GA or EDD, depending on the model) that is not part of the dataset used to create the model (i.e., regression line/curve, etc.). Estimation accounts for uncertainty in the regression line/curve (i.e., the model). Prediction accounts for uncertainty in the regression line/curve (i.e., the model) plus the uncertainty in the individual observation (e.g., an individual ultrasound fetal metric measurement). Therefore, since both estimation and prediction account for uncertainty in the regression line or curve (i.e. the model), some slack will be cut and both terms, "estimation" and "prediction" will be used in reference to model-derived predictions/estimations (e.g., GA or EDD predictions/estimations) made from predictors (i.e., fetal metric measurements: CRL, HC, BPD, FL, AC, MAD, etc.)
Given that the terms "estimation" and "prediction" have been conflated and used extensively as synonyms there are those who would similarly conflate other terms such as "estimate" and "calculate." For example, NCFM eSnurra Group intentionally conflate the "prediction/estimation" of GA with the "prediction/estimation" of EDD in order to obfuscate the differences between these 2 completely different methods of establishing GA and EDD, respectively, for individual pregnancies. Moreover, NCFM eSnurra Group then describes these methods as being synonymous to healthcare authorities, healthcare professionals and the general public when, in reality, these 2 separate prediction/estimation methods have distinct differences and each with very different "prediction/estimation" objectives. This science-bending, conflate-to-obfuscate strategy for GA and EDD prediction/estimation is a real-world example of bending policy-relevant science; and, unfortunately, in this example Norway's national obstetric & fetal medicine policy was bent to cause increased medical risks, critical medical mistakes and grievous medical harms (including perinatal death), unnecessarily, for some of Norway's women and their fetuses/babies.
- White Paper on Publication Ethics (Download a PDF of the entire White Paper)
Dates All dates are in dd.mm.yyyy format unless otherwise indicated.
Death
- Infant death death <1 year of life
- Perinatal death stillbirth or death <=6 completed days of life
- Neonatal death death >6 and <=27 completed days of life; Neonatal Mortality Rate (NMR)
- Post-neonatal death death >= 28th completed day of life and <= 1 completed year of life
Dolichocephaly, more commonly "long head" or "breech head"; Cephalic index (CI) <= 75
Doublethink "Doublethink is the act of simultaneously accepting two mutually contradictory beliefs as correct, often in distinct social contexts. [1] Doublethink is related to, but differs from, hypocrisy and neutrality. Also related is cognitive dissonance, in which contradictory beliefs cause conflict in one's mind. Doublethink is notable due to a lack of cognitive dissonance—thus the person is completely unaware of any conflict or contradiction." (Source: Wikipedia: Doublethink)
- "DOUBLETHINK means the power of holding two contradictory beliefs in one’s mind simultaneously, and accepting both of them. The Party intellectual knows in which direction his memories must be altered; he therefore knows that he is playing tricks with reality; but by the exercise of DOUBLETHINK he also satisfies himself that reality is not violated. The process has to be conscious, or it would not be carried out with sufficient precision, but it also has to be unconscious, or it would bring with it a feeling of falsity and hence of guilt" (Source: George Orwell, "1984" Part Two, Chapter 9)
- "It was as though some huge force were pressing down upon you — something that penetrated inside your skull, battering against your brain, frightening you out of your beliefs, persuading you, almost, to deny the evidence of your senses. In the end the Party would announce that two and two made five, and you would have to believe it. It was inevitable that they should make that claim sooner or later: the logic of their position demanded it. Not merely the validity of experience, but the very existence of external reality, was tacitly denied by their philosophy. The heresy of heresies was common sense." (Source: George Orwell, "1984" Part One, Chapter 7)
- "Freedom is the freedom to say that two plus two makes four. If that is granted, all else follows" (Source: ibid.)
Estimation vs. Prediction
The terms "estimation" and "prediction" have been conflated and and are commonly used interchangeably as synonyms in the ultrasound biometry literature, which is unfortunate. Consequently, this document will also use these terms somewhat interchangeably. However, in classical statistical inference there is a clear distinction between an estimator and predictor. An estimator uses data to guess at a parameter or coefficient while a predictor (e.g., an ultrasound fetal metric measurement) uses data to guess at some random value (e.g., GA or EDD, depending on the model) that is not part of the dataset used to create the model (i.e., regression line/curve, etc.). Estimation accounts for uncertainty in the regression line/curve (i.e., the model). Prediction accounts for uncertainty in the regression line/curve (i.e., the model) plus the uncertainty in the individual observation (e.g., an individual ultrasound fetal metric measurement). Therefore, since both estimation and prediction account for uncertainty in the regression line or curve (i.e. the model), some slack will be cut and both terms, "estimation" and "prediction" will be used in reference to model-derived predictions/estimations (e.g., GA or EDD predictions/estimations) made from predictors (i.e., fetal metric measurements: CRL, HC, BPD, FL, AC, MAD, etc.)
Given that the terms "estimation" and "prediction" have been conflated and used extensively as synonyms there are those who would similarly conflate other terms such as "estimate" and "calculate." For example, NCFM eSnurra Group intentionally conflate the "prediction/estimation" of GA with the "prediction/estimation" of EDD in order to obfuscate the differences between these 2 completely different methods of establishing GA and EDD, respectively, for individual pregnancies. Moreover, NCFM eSnurra Group then describes these methods as being synonymous to healthcare authorities, healthcare professionals and the general public when, in reality, these 2 separate prediction/estimation methods have distinct differences and each with very different "prediction/estimation" objectives. This science-bending, conflate-to-obfuscate strategy for GA and EDD prediction/estimation is a real-world example of bending policy-relevant science; and, unfortunately, in this example Norway's national obstetric & fetal medicine policy was bent to cause increased medical risks, critical medical mistakes and grievous medical harms (including perinatal death), unnecessarily, for some of Norway's women and their fetuses/babies.
Ethics in Scientific Research
"Ethics in Scientific Research: An Examination of Ethical Principles and Emerging Topics"
"Key Findings
This research found ten ethical principles common across scientific disciplines
"Ethics in Scientific Research: An Examination of Ethical Principles and Emerging Topics"
"Key Findings
This research found ten ethical principles common across scientific disciplines
- They are duty to society; beneficence; conflict of interest; informed consent; integrity; nondiscrimination; nonexploitation; privacy and confidentiality; professional competence; and professional discipline. [See Table S.1 below]
- Each ethical principle applies to the scientific inquiry, the conduct and behaviors of researchers, or the ethical treatment of research participants.
- Only one ethical principle — duty to society — applies to the scientific inquiry by asking whether the research benefits society.
- Variations in ethical principles across disciplines are usually due to whether the discipline includes human or animal subjects.
- Variations in ethical principles across countries are usually due to local laws, oversight, and enforcement; cultural norms; and whether research is conducted in the researchers' host country or a foreign country.
- significant historic events that create a reckoning
- ethical lapses that lead researchers to create new safeguards
- scientific advancements that lead to new fields of research
- changes in cultural values and behavioral norms that evolve over time.
- Professional societies and peer-reviewed journals offer consistent ethical standards across national borders, though they lack the enforcement strength of nation-states.
- Emerging trends — including big data, open science, and citizen science — provide research opportunities while introducing new ethical risks.
- Professional societies respond to emerging changes with updates to codes of conduct, education and training for researchers, and governance structures for researchers, sponsors, and research subjects. (Source: "Ethics in Scientific Research: An Examination of Ethical Principles and Emerging Topics" by Cortney Weinbaum, Eric Landree, Marjory S. Blumenthal, Tepring Piquado, Carlos Ignacio Gutierrez. Santa Monica, CA: RAND Corporation, 2019. ISBN: 978-1-9774-0269-1. https://www.rand.org/pubs/research_reports/RR2912.html. View/Download pdf here.)
Table S.1 (Source: Ibid., p. x)
Ethical Principles for Scientific Research
Ethical Principles for Scientific Research
Ethical Principle |
Definition |
Duty to society |
Researchers and research must contribute to the well-being of society. |
Beneficence |
Researchers should have the welfare of the research participant in mind as a goal and strive for the benefits of the research to outweigh the risks. |
Conflict of interest |
Researchers should minimize financial and other influences on their research and on research participants that could bias research results. Conflict of interest is more frequently directed at the researcher, but it may also involve the research participants if they are provided with a financial or nonfinancial incentive to participate. |
Informed consent |
All research participants must voluntarily agree to participate in research, without pressure from financial gain or other coercion, and their agreement must include an understanding of the research and its risks. When participants are unable to consent or when vulnerable groups are involved in research, specific actions must be taken by researchers and their institutions to protect the participants. |
Integrity |
Researchers should demonstrate honesty and truthfulness. They should not fabricate data, falsify results, or omit relevant data. They should report findings fully, minimize or eliminate bias in their methods, and disclose underlying assumptions |
Nondiscrimination |
Researchers should minimize attempts to reduce the benefits of research on specific groups and to deny benefits from other groups. |
Nonexploitation |
Researchers should not exploit or take unfair advantage of research participants. |
Privacy and confidentiality |
Privacy: Research participants have the right to control access to their personal information and to their bodies in the collection of biological specimens. Participants may control how others see, touch, or obtain their information. Confidentiality: Researchers will protect the private information provided by participants from release. Confidentiality is an extension of the concept of privacy; it refers to the participant’s understanding of, and agreement to, the ways identifiable information will be stored and shared. |
Professional competence |
Researchers should engage only in work that they are qualified to perform, while also participating in training and betterment programs with the intent of improving their skill sets. This concept includes how researchers choose research methods, statistical methods, and sample sizes that are appropriate and would not cause misleading results. |
Professional discipline |
Researchers should engage in ethical research and help other researchers engage in ethical research by promulgating ethical behaviors through practice, publishing and communicating, mentoring and teaching, and other activities. |
NOTE: Research participant refers to someone with an active role participating in research, whereas research subject could include someone whose data are used but who does not consent to participate.
Ethics in Scientific Research (continued)
Unethical laws & unregulated ethics...not enforceable & often not even monitored
Unethical laws & unregulated ethics...not enforceable & often not even monitored
- "As we examined laws, regulations, and standards around the world, we sought an understanding of the relationship between enforceable laws and unenforceable norms. We undertook this project with an understanding that laws can be unethical and ethics can be unregulated, and we hoped to understand how the administration of both laws and ethics may better align. We found that professional societies and journals aim to fill the gap between laws and ethics by documenting ethics that they expect of their members or authors, respectively; requiring members and authors to self-certify that they complied with such rules; and providing a reporting or grievance mechanism for cases in which members or authors self-report or are reported on. When these societies or journals have international membership or readership, they additionally seek to smooth out ethical differences from region to region by creating a discipline-wide standard. These mechanisms are imperfect, however, as they are not enforceable and often not even monitored." (Source: Ibid., p. xi-xii)
Evidence-based medicine (EBM)
"Evidence based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research. By individual clinical expertise we mean the proficiency and judgment that individual clinicians acquire through clinical experience and clinical practice. Increased expertise is reflected in many ways, but especially in more effective and efficient diagnosis and in the more thoughtful identification and compassionate use of individual patients' predicaments, rights, and preferences in making clinical decisions about their care." (Source: "Evidence based medicine: what it is and what it isn't" David L Sackett, William M C Rosenberg, J A Muir Gray, R Brian Haynes, W Scott Richardson. BMJ 1996;312:71. https://doi.org/10.1136/bmj.312.7023.71. Published 13 January 1996)
"Evidence based medicine is not “cookbook” medicine. Because it requires a bottom up approach that integrates the best external evidence with individual clinical expertise and patients' choice, it cannot result in slavish, cookbook approaches to individual patient care. External clinical evidence can inform, but can never replace, individual clinical expertise, and it is this expertise that decides whether the external evidence applies to the individual patient at all and, if so, how it should be integrated into a clinical decision. Similarly, any external guideline must be integrated with individual clinical expertise in deciding whether and how it matches the patient's clinical state, predicament, and preferences, and thus whether it should be applied. Clinicians who fear top down cookbooks will find the advocates of evidence based medicine joining them at the barricades." (Source: ibid.)
"There is an illusion that the ability to synthesise evidence provides all of the knowledge needed to practice medicine: that one has only to read the literature, or to adopt the guideline, to gain a mastery of the field. Governments, policy makers and payers have been seduced into believing that the rationalist EBM paradigm holds the solution to, for example, the task of providing high-quality medical care to an ageing population with complex needs. There is a push to codify and regulate the practice of medicine, to apply guidelines, and to pay for performance to standardise care, in the hope that this approach to evidence translation will reduce cost and improve outcomes that are of importance to institutions; Sackett warned of the risk of evidence-based medicine being hijacked to this purpose in 1996." (Source: "The 11th hour-time for EBM to return to first principles?" Denise Campbell-Scherer. BMJEvidence-Based Medicine 2012;17:103-104. DOI: 10.1136/ebmed-2012-100578. Pubmed ID22736667.)
"Evidence-based medicine is the conscientious use of current best evidence in making decisions about the care of individual patients or the delivery of health services. The terms ‘evidence-based health care’ and ‘evidence-based practice’ are often used interchangeably with ‘evidence-based medicine’" (Source: "SUPPORT Tools for evidence-informed health Policymaking (STP)" Report from Norwegian Knowledge Centre for the Health Services No 4–2010, Chapter 1, p. 16-25. ISBN print 978-82-8121-313-5, ISBN digital 978-82-8121-334-0, ISSN 1890-1298)
Evidence-informed health policymaking (EIHP) "evidence-informed health policymaking is an approach to policy decisions that aims to ensure that decision making is well-informed by the best available research evidence. It is characterised by the systematic and transparent access to, and appraisal of, evidence as an input into the policymaking process" (Source: ibid., p. 16-25)
Fetal Age Fetal age is the actual age of the developing and growing fetus/baby from the day of conception. Gestational age is the age of the pregnancy from the first day of the last normal menstrual period (LMP).
Fetal Biometry (also known as fetometery) "Fetal biometric parameters are antenatal ultrasound measurements that are used to indirectly assess the growth and well being of the fetus." (Source: Radiopedia: fetal metrics)
"Evidence based medicine is the conscientious, explicit, and judicious use of current best evidence in making decisions about the care of individual patients. The practice of evidence based medicine means integrating individual clinical expertise with the best available external clinical evidence from systematic research. By individual clinical expertise we mean the proficiency and judgment that individual clinicians acquire through clinical experience and clinical practice. Increased expertise is reflected in many ways, but especially in more effective and efficient diagnosis and in the more thoughtful identification and compassionate use of individual patients' predicaments, rights, and preferences in making clinical decisions about their care." (Source: "Evidence based medicine: what it is and what it isn't" David L Sackett, William M C Rosenberg, J A Muir Gray, R Brian Haynes, W Scott Richardson. BMJ 1996;312:71. https://doi.org/10.1136/bmj.312.7023.71. Published 13 January 1996)
"Evidence based medicine is not “cookbook” medicine. Because it requires a bottom up approach that integrates the best external evidence with individual clinical expertise and patients' choice, it cannot result in slavish, cookbook approaches to individual patient care. External clinical evidence can inform, but can never replace, individual clinical expertise, and it is this expertise that decides whether the external evidence applies to the individual patient at all and, if so, how it should be integrated into a clinical decision. Similarly, any external guideline must be integrated with individual clinical expertise in deciding whether and how it matches the patient's clinical state, predicament, and preferences, and thus whether it should be applied. Clinicians who fear top down cookbooks will find the advocates of evidence based medicine joining them at the barricades." (Source: ibid.)
"There is an illusion that the ability to synthesise evidence provides all of the knowledge needed to practice medicine: that one has only to read the literature, or to adopt the guideline, to gain a mastery of the field. Governments, policy makers and payers have been seduced into believing that the rationalist EBM paradigm holds the solution to, for example, the task of providing high-quality medical care to an ageing population with complex needs. There is a push to codify and regulate the practice of medicine, to apply guidelines, and to pay for performance to standardise care, in the hope that this approach to evidence translation will reduce cost and improve outcomes that are of importance to institutions; Sackett warned of the risk of evidence-based medicine being hijacked to this purpose in 1996." (Source: "The 11th hour-time for EBM to return to first principles?" Denise Campbell-Scherer. BMJEvidence-Based Medicine 2012;17:103-104. DOI: 10.1136/ebmed-2012-100578. Pubmed ID22736667.)
"Evidence-based medicine is the conscientious use of current best evidence in making decisions about the care of individual patients or the delivery of health services. The terms ‘evidence-based health care’ and ‘evidence-based practice’ are often used interchangeably with ‘evidence-based medicine’" (Source: "SUPPORT Tools for evidence-informed health Policymaking (STP)" Report from Norwegian Knowledge Centre for the Health Services No 4–2010, Chapter 1, p. 16-25. ISBN print 978-82-8121-313-5, ISBN digital 978-82-8121-334-0, ISSN 1890-1298)
Evidence-informed health policymaking (EIHP) "evidence-informed health policymaking is an approach to policy decisions that aims to ensure that decision making is well-informed by the best available research evidence. It is characterised by the systematic and transparent access to, and appraisal of, evidence as an input into the policymaking process" (Source: ibid., p. 16-25)
Fetal Age Fetal age is the actual age of the developing and growing fetus/baby from the day of conception. Gestational age is the age of the pregnancy from the first day of the last normal menstrual period (LMP).
Fetal Biometry (also known as fetometery) "Fetal biometric parameters are antenatal ultrasound measurements that are used to indirectly assess the growth and well being of the fetus." (Source: Radiopedia: fetal metrics)
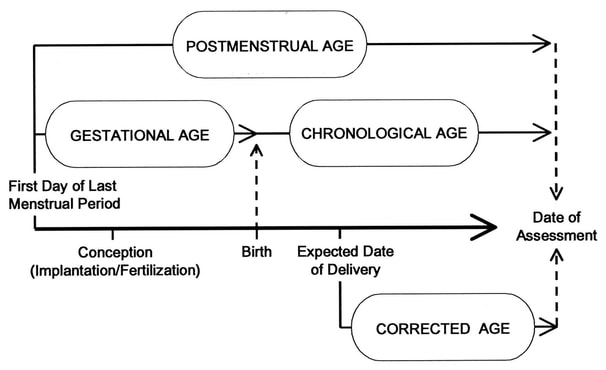
Gestational Age (GA) & Age Terminology
"Age Terminology During the Perinatal Period"
"Gestational age is often determined by the “best obstetric estimate,” which is based on a combination of the first day of last menstrual period, physical examination of the mother, prenatal ultrasonography, and history of assisted reproduction. The best obstetric estimate is necessary because of gaps in obstetric information and the inherent variability (as great as 2 weeks) in methods of gestational age estimation. 8,10,14–19 Postnatal physical examination of the infant is sometimes used as a method to determine gestational age if the best obstetric estimate seems inaccurate. Therefore, methods of determining gestational age should be clearly stated so that the variability inherent in these estimations can be considered when outcomes are interpreted. 8,10,14–19
RECOMMENDATIONS
1. Standardized terminology should be used when defining ages and comparing outcomes of fetuses and newborns. The recommended terms (Table 1) are:
3. “Conceptional age,” “postconceptional age,” “conceptual age,” and “postconceptual age” should not be used in clinical pediatrics.
4. Publications reporting fetal and neonatal outcomes should clearly describe methods used to
determine gestational age."
"Age Terminology During the Perinatal Period"
"Gestational age is often determined by the “best obstetric estimate,” which is based on a combination of the first day of last menstrual period, physical examination of the mother, prenatal ultrasonography, and history of assisted reproduction. The best obstetric estimate is necessary because of gaps in obstetric information and the inherent variability (as great as 2 weeks) in methods of gestational age estimation. 8,10,14–19 Postnatal physical examination of the infant is sometimes used as a method to determine gestational age if the best obstetric estimate seems inaccurate. Therefore, methods of determining gestational age should be clearly stated so that the variability inherent in these estimations can be considered when outcomes are interpreted. 8,10,14–19
RECOMMENDATIONS
1. Standardized terminology should be used when defining ages and comparing outcomes of fetuses and newborns. The recommended terms (Table 1) are:
- Gestational age (completed weeks): time elapsed between the first day of the last menstrual period and the day of delivery. If pregnancy was achieved using assisted reproductive technology, gestational age is calculated by adding 2 weeks to the conceptional age.
- Chronological age (days, weeks, months, or years): time elapsed from birth.
- Postmenstrual age (weeks): gestational age plus chronological age.
- Corrected age (weeks or months): chronological age reduced by the number of weeks born before 40 weeks of gestation; the term should be used only for children up to 3 years of age who were born preterm.
3. “Conceptional age,” “postconceptional age,” “conceptual age,” and “postconceptual age” should not be used in clinical pediatrics.
4. Publications reporting fetal and neonatal outcomes should clearly describe methods used to
determine gestational age."
Figure 1. Age terminology during the perinatal period.
(Source: "Age Terminology During the Perinatal Period" Pediatrics November 2004, VOLUME 114 / ISSUE 5
AMERICAN ACADEMY OF PEDIATRICS, POLICY STATEMENT, Organizational Principles to Guide and Define the Child Health Care System and/or Improve the Health of All Children, Committee on Fetus and Newborn. View/Download PDF here) [A Statement Of Reaffirmation For This Policy Was Published At 123(1):188 127(5):e1367 123(5):1421 134(3):e920; Policy Statement: Age Terminology During the Perinatal Period. Pediatrics. 2004;114(5):1362–1364. Reaffirmed July 2014]
AMERICAN ACADEMY OF PEDIATRICS, POLICY STATEMENT, Organizational Principles to Guide and Define the Child Health Care System and/or Improve the Health of All Children, Committee on Fetus and Newborn. View/Download PDF here) [A Statement Of Reaffirmation For This Policy Was Published At 123(1):188 127(5):e1367 123(5):1421 134(3):e920; Policy Statement: Age Terminology During the Perinatal Period. Pediatrics. 2004;114(5):1362–1364. Reaffirmed July 2014]
Gestational Age (GA) Notation & Math(s)
Gestational weeks are expressed as completed weeks. Gestational days are expressed as completed days.
Goodhart’s law
"Watch out for cheats in citation game"
Harm Principle (skadefølgeprinsippet)
Research Ethics Act
Health Systems & Policy Monitor (HSPM)
"The European Observatory on Health Systems and Policies supports and promotes evidence-based health policy-making through comprehensive and rigorous analysis of the dynamics of health care systems in Europe."
Health Technology Assessment (HTA)
Gestational weeks are expressed as completed weeks. Gestational days are expressed as completed days.
- For example, day 3 of completed week 17 is expressed as: 17+3 or 17w+3 or 17w+3d or 17/3 or 17+3/7
- The first day of completed week 17 is expressed as: 17+0
- The last day of completed week 17 is expressed as 17+6; [Note: 17+7 is never valid; XXw+7 is never valid; XX+7/7 is never valid]
- The last day of completed week 17 is 17w+6
- The day after 17w+6 is 18w+0, which is the first completed day of completed week 18.
- GA in completed days: 17w+3 = (17 weeks x 7 days/week) + 3 days = (119 days) + 3 days = 122 days
Goodhart’s law
"Watch out for cheats in citation game"
- "All metrics of scientific evaluation are bound to be abused. Goodhart’s law (named after the British economist who may have been the first to announce it) states that when a feature of the economy is picked as an indicator of the economy, then it inexorably ceases to function as that indicator because people start to game it.
What we see today, however, is not just the gaming of science metrics indicators, but the emergence of a new kind of metrics-enabled fraud, which we can call post-production misconduct. It seems to be as widespread as other forms, with at least 300 papers already retracted because the peer review had been tampered with.
(...)
Given the increasing awareness of post-production misconduct— and how it undermines the assessment of publicly funded research — funders, policymakers and the science community should ask publishers to make available more of the information needed to investigate it.
The community must realize that, unlike previous fraudsters, from the unknown hoaxer who planted a mixture of bones in a British gravel pit at Piltdown to that of Paul Kammerer, who is blamed for inkingfeatures onto the feet of midwife toads to support Lamarckianism evolution, academic misconduct is no longer just about seeking attention. Many academic fraudsters aren’t aiming for a string of high-profile publications. That’s too risky. They want to produce — by plagiarism and rigging the peer-review system — publications that are near invisible, but can give them the kind of curriculum vitae that matches the performance metrics used by their academic institutions. They aim high, but not too high.
And so do their institutions — typically not the world’s leading universities, but those that are trying to break into the top rank. These are the institutions that use academic metrics most enthusiastically, and so end up encouraging post-production misconduct. The audit culture of universities — their love affair with metrics, impact factors, citation statistics and rankings — does not just incentivize this new form of bad behaviour. It enables it." (Source: "Watch out for cheats in citation game" "The focus on impact of published research has created new opportunities for misconduct and fraudsters, says Mario Biagioli." Nature 535, 201 (14 July 2016) doi:10.1038/535201a)
- "Intentional performance of an unreasonable act in disregard of a known risk, making it highly probable that harm will be caused. Willful negligence usually involves a conscious indifference to the consequences. There is no clear distinction between willful negligence and gross negligence." (Source: Legal Glossary: willful negligence) Also: "the type of negligence that is deliberate with the intentional disregard for other people's welfare." (Source: The Law Dictionary: Black's Law Dictionary: willful negligence)
Harm Principle (skadefølgeprinsippet)
Research Ethics Act
- "3.2. The Harm Principle as Guidance for Criminalisation
In light of this pragmatic tradition, a strongly principled statement on criminalisation was somewhat surprisingly given in NOU 2002: 4. Here, the harm principle (skadefølgeprinsippet) was adopted and applied as a principal starting point for the criminal code. The code of 2005 is based on the idea that punishment should solely be used as a reaction towards actions that lead to, or could lead to, harm being inflicted on someone. 24 The idea is that a specific behaviour should not be criminalised only because the majority dislikes it. The use of punishment must be rational and humane. 25 Punishment should only be used in cases where compelling reasons justify it, and actions that lead to the harm of people or assets will typically constitute such compelling reasons. The harm principle was the fundamental starting point for the work of the Legislative Commission of the Criminal Code. 26" (Source: "An Outline of the New Norwegian Criminal Code" JØRN JACOBSEN & VILDE HALLGREN SANDVIK. Bergen Journal of Criminal Law and Criminal Justice • Volume 3, Issue 2, 2015, pp. 162-183)
Citation References [links added]
24 This was set out in NOU 2002: 4, see in particular pp. 79-81.
25 Ot.prp. nr. 90 (2003-2004) p. 89
26 NOU 2002: 4, p. 79.
Health Systems & Policy Monitor (HSPM)
"The European Observatory on Health Systems and Policies supports and promotes evidence-based health policy-making through comprehensive and rigorous analysis of the dynamics of health care systems in Europe."
- HSPM: http://www.hspm.org/
- Norway's Health Care System: http://www.hspm.org/countries/norway08012014/countrypage.aspx
(Source: http://www.euro.who.int/en/about-us/partners/observatory/about-us)
Health Technology Assessment (HTA)
- The Norwegian Directorate of Health (Helsedirectoratet) defines Health Technology Assessment (HTA) as follows.
"HTA Health Technology Assessment. Tools that will help to make knowledge-based decisions when introducing new methods in the health service. Should elucidate the effects and safety-related, health-economic, ethical, legal and social consequences of the method"
"HTA Health Technology Assessment. Vektøy som skal bidra til å ta kunnskapsbaserte beslutninger ved innføring av nye metoder i helsetjenesten. Bør belyse effekt og sikkerhetsmessige, helseøkonomiske, etiske, juridiske og sosiale konsekvenser knyttet til metoden" (Source: "Guide for the development of knowledge-based guidelines" ("Veileder for utvikling av kunnskapsbaserte retningslinjer") Redaktør: Caroline Hodt-Billington. Utgitt: 10/2012. Publikasjonsnummer: IS-1870. ISBN-nr. 978-82-8081-225-4. Utgitt av: Helsedirektoratet, 2012. p. 7)
View/Download Norwegian Directorate of Health's 2-page "Checklist for the development of knowledge-based guidelines" (Sjekkliste for utvikling av kunnskapsbaserte retningslinjer) View/Download Norwegian Directorate of Health's 58-page "Guide for the development of knowledge-based guidelines" (Veileder for utvikling av kunnskapsbaserte retningslinjer) (Source: Helsedirectoratet website: https://www.helsedirektoratet.no/veiledere/utvikling-av-kunnskapsbaserte-retningslinjer)
- "What is Health Technology Assessment (HTA)?
HTA is the systematic evaluation of the properties and effects of a health technology, addressing the direct and intended effects of this technology, as well as its indirect and unintended consequences, and aimed mainly at informing decision making regarding health technologies. HTA is conducted by interdisciplinary groups that use explicit analytical frameworks drawing on a variety of methods." (Source: "Welcome to INAHTA" INAHTA Website: http://www.inahta.org/)
"A health technology is defined as an intervention that may be used to promote health, to prevent, diagnose or treat acute or chronic disease, or for rehabilitation. Health technologies include pharmaceuticals, devices, procedures and organizational systems used in health care." (Source: ibid.)
"For more definitions, see the International HTA Glossary." (Source: ibid.) - "Health Technology Assessment • [HTA] is a multidisciplinary field of policy analysis. It studies the medical, social, ethical, and economic implications of development, diffusion, and use of health technology (International Network of Agencies for Health Technology Assessment 2002)" (Source: "Health Technology Assessment of innovative medical devices" Dr. Iñaki Gutiérrez-Ibarluzea, Knowledge Manager, Vicepresident of EuroScan International Network. Third WHO Global Forum on Medical Devices 10-12 May 2017)
Impact Factor (IF) "The impact factor or journal impact factor of an academic journal is a scientometric index which reflects the yearly average number of citations to recent articles published in that journal." (Source: https://en.wikipedia.org/wiki/Impact_factor)
Impact Factor is a non-level playing field for journal rankings
"How a Single Paper Affects the Impact Factor: Implications for Scholarly Publishing"
Impact Factor is a non-level playing field for journal rankings
"How a Single Paper Affects the Impact Factor: Implications for Scholarly Publishing"
- "Abstract
Because the Impact Factor (IF) is an average quantity and most journals are small, IFs are volatile. We study how a single paper affects the IF using data from 11639 journals in the 2017 Journal Citation Reports. We define as volatility the IF gain (or loss) caused by a single paper, and this is inversely proportional to journal size. We find high volatilities for hundreds of journals annually due to their top-cited paper—whether it is a highly-cited paper in a small journal, or a moderately (or even low) cited paper in a small and low-cited journal. For example, 1218 journals had their most cited paper boost their IF by more than 20%, while for 231 journals the boost exceeded 50%. We find that small journals are rewarded much more than large journals for publishing a highly-cited paper, and are also penalized more for publishing a low-cited paper, especially if they have a high IF. This produces a strong incentive for prestigious, high-IF journals to stay small, to remain competitive in IF rankings. We discuss the implications for breakthrough papers to appear in prestigious journals. We also question the practice of ranking journals by IF given this uneven reward mechanism. (Source: "How a Single Paper Affects the Impact Factor: Implications for Scholarly Publishing" Manolis Antonoyiannakis, Department of Applied Physics & Applied Mathematics, Columbia University; Associate Editor at the American Physical Society . arXiv preprint arXiv:1906.02660, 2019. https://arxiv.org/ftp/arxiv/papers/1906/1906.02660.pdf) - "Conclusions
The above findings corroborate our earlier conclusion (Antonoyiannakis, 2018) that IFs are scale dependent and particularly volatile to small journal sizes, as explained by the Central Limit Theorem. This point is pertinent for real journals because 90% of all journals publish no more than 250 citable items annually (Antonoyiannakis, 2018).
Compared to large journals, small journals have (a) much more to gain by publishing a highlycited paper, and (b) more to lose by publishing a little-cited paper—that is, more to gain by rejecting a little-cited paper. Therefore, in terms of IF, it pays for small journals to be selective.
The fact that there are more than a hundred journals annually whose highest cited paper suffices to raise their citation average by 1 point demonstrates that the effect we study here is not of academic but of practical interest. If so many journals are that much affected by a single paper, then the usefulness of the IF as a journal defining quantity is questioned, as is the practice of IF rankings of journals. This point becomes even more pertinent when we consider the relative volatility. Evidently (see Table 4), for 1 out of 50 journals (231 journals) a single paper boosts the IF by 50%. Roughly 1 out of 10 journals (1218 journals) had their IF boosted by more than 20% by a single paper. And for more than a quarter of all journals (3403 journals) the IF increased more than 10% by a single paper.
So, the IF volatility affects thousands of journals. It is not an exclusive feature of a few journals or a statistical anomaly that we can casually brush off, but an everyday feature that is inherent in citation averages (IFs) and affects many journals, every year.
The high volatility of IF values from real-journal data demonstrates that ranking journals by IFs constitutes a non-level playing field, since the IF gain of publishing an equally cited paper scales as the inverse journal size and can therefore span up to 4 orders of magnitude across journals. It is therefore critical to consider novel ways of comparing journals based on more solid statistical grounds. The implications of such a decision may reach much further than producing ranked journal lists aimed at librarians (the original motivation for the IF) and affect research assessment and the careers or scientists." (Source: Ibid.; Also see: (Antonoyiannakis, 2018) or "Impact Factors and the Central Limit Theorem: Why Citation Averages Are Scale Dependent" Manolis Antonoyiannakis, Department of Applied Physics & Applied Mathematics, Columbia University; Associate Editor at the American Physical Society. arXiv preprint arXiv:1803.02436v3 [physics.soc-ph] 20 Sep 2018. https://arxiv.org/pdf/1803.02436.pdf)
Medical Mistake or Medical Error "Medical error has been defined as an unintended act (either of omission or commission) or one that does not achieve its intended outcome, 3 the failure of a planned action to be completed as intended (an error of execution), the use of a wrong plan to achieve an aim (an error of planning), 4 or a deviation from the process of care that may or may not cause harm to the patient. 5" (Source: "Medical error—the third leading cause of death in the US" Martin A Makary & Michael Daniel. BMJ 2016;353:i2139; or the PDF version)
Meta-analysis is a statistical technique used to pool results from multiple individual studies, usually 6 or more individual studies. For example:
Moral Disengagement
"In this paper, we propose that an important additional driver of unethical behavior is an individual’s propensity to morally disengage—that is, an individual difference in the way that people cognitively process decisions and behavior with ethical import that allows those inclined to morally disengage to behave unethically without feeling distress (Bandura, 1990a, 1990b, 1999, 2002). Broadly speaking, we know that how individuals processs, frame, or understand information relevant to ethically meaningful decisions plays an important role in their ethical and unethical choices (Kern & Chugh, 2009; Tenbrunsel & Messick, 1999), and recent reviews of the behavioral ethics literature have suggested that scholars should attend more carefully to the role of cognitive processes in unethical behavior (Tenbrunsel & Messick, 2004; Tenbrunsel & SmithCrowe, 2008). In the set of studies reported here, we heed these calls by establishing a new measure of the propensity to morally disengage as a uniquely important predictor of a broad range of unethical behaviors." (Source: "WHY EMPLOYEES DO BAD THINGS: MORAL DISENGAGEMENT AND UNETHICAL ORGANIZATIONAL BEHAVIOR" MOORE, C., DETERT, J. R., KLEBE TREVIÑO, L., BAKER, V. L., & MAYER, D. M. (2012) Personnel Psychology, 65(1), 1–48. doi:10.1111/j.1744-6570.2011.01237.x)
Naegele's rule is a 200+ year-old algorithm or 'rule' used to calculate the expected date/day of delivery (EDD) of a pregnancy from the first day of a woman's last menstrual period (LMP) date; this is done by adding a year, subtracting three months, and adding seven days. The result is approximately 280 days (or 40 weeks) from the first day of the last menstrual period. Naegele's rule was originally formulated by Hermanni Boerhaave, however, Naegele popularized it (while giving full attribution to Boerhaave) and it became known as Naegele's rule. (Source: "Naegele’s rule: a reappraisal")
Obviate "to anticipate and prevent (as a situation) or make unnecessary (as an action), to make (something) no longer necessary : to prevent or avoid (something) Obviate has a number of synonyms in English, including "prevent," "preclude," and "avert"; all of these words can mean to hinder or stop something. When you prevent or preclude something, you put up
an insurmountable obstacle." (Source: Merriam-Webster: obviate)
Peer Review
"Ethics of Peer Review: A Guide for Manuscript Reviewers"
"COPE Ethical Guidelines for Peer Reviewers - English"
"Peer-Review Fraud — Hacking the Scientific Publication Process"
Meta-analysis is a statistical technique used to pool results from multiple individual studies, usually 6 or more individual studies. For example:
- "Similar issues arise in some epidemiological studies—eg, had investigators researching possible aetiological factors in sudden infant death syndrome taken proper account of what was already known, the lethal effect of babies lying on their front would have been recognised at least a decade earlier than it was, and tens of thousands of infant deaths could have been avoided. 78 Similarly, a cumulative meta-analysis 79 of 55 studies done in a 24 year period showed that the research had repeatedly confirmed that never-smoking women who had been exposed to smoking via their husbands were more likely than others were to develop lung cancer." (Source: "How to increase value and reduce waste when research priorities are set" Iain Chalmers, Michael B Bracken, Ben Djulbegovic, Silvio Garattini, Jonathan Grant, A Metin Gülmezoglu, David W Howells, John P A Ioannidis, Sandy Oliver. The Lancet 2014; 383: 156–65 January 11, 2014. Published Online January 8, 2014 http://dx.doi.org/10.1016/S0140-6736(13)62229-1)
Moral Disengagement
"In this paper, we propose that an important additional driver of unethical behavior is an individual’s propensity to morally disengage—that is, an individual difference in the way that people cognitively process decisions and behavior with ethical import that allows those inclined to morally disengage to behave unethically without feeling distress (Bandura, 1990a, 1990b, 1999, 2002). Broadly speaking, we know that how individuals processs, frame, or understand information relevant to ethically meaningful decisions plays an important role in their ethical and unethical choices (Kern & Chugh, 2009; Tenbrunsel & Messick, 1999), and recent reviews of the behavioral ethics literature have suggested that scholars should attend more carefully to the role of cognitive processes in unethical behavior (Tenbrunsel & Messick, 2004; Tenbrunsel & SmithCrowe, 2008). In the set of studies reported here, we heed these calls by establishing a new measure of the propensity to morally disengage as a uniquely important predictor of a broad range of unethical behaviors." (Source: "WHY EMPLOYEES DO BAD THINGS: MORAL DISENGAGEMENT AND UNETHICAL ORGANIZATIONAL BEHAVIOR" MOORE, C., DETERT, J. R., KLEBE TREVIÑO, L., BAKER, V. L., & MAYER, D. M. (2012) Personnel Psychology, 65(1), 1–48. doi:10.1111/j.1744-6570.2011.01237.x)
Naegele's rule is a 200+ year-old algorithm or 'rule' used to calculate the expected date/day of delivery (EDD) of a pregnancy from the first day of a woman's last menstrual period (LMP) date; this is done by adding a year, subtracting three months, and adding seven days. The result is approximately 280 days (or 40 weeks) from the first day of the last menstrual period. Naegele's rule was originally formulated by Hermanni Boerhaave, however, Naegele popularized it (while giving full attribution to Boerhaave) and it became known as Naegele's rule. (Source: "Naegele’s rule: a reappraisal")
- "Naegele's rule is a simple method of pregnancy dating. The EDD is calculated by counting back three months from the LMP and adding seven days. As an example, if the LMP is February 20, then the EDD will be November 27. If the LMP is May 28, then the EDD will be March 4. This method assumes the patient has a 28-day menstrual cycle with fertilization occurring on day 14." (Source: UpToDate.com: https://www.uptodate.com/contents/prenatal-assessment-of-gestational-age-and-estimated-date-of-delivery)
Obviate "to anticipate and prevent (as a situation) or make unnecessary (as an action), to make (something) no longer necessary : to prevent or avoid (something) Obviate has a number of synonyms in English, including "prevent," "preclude," and "avert"; all of these words can mean to hinder or stop something. When you prevent or preclude something, you put up
an insurmountable obstacle." (Source: Merriam-Webster: obviate)
Peer Review
"Ethics of Peer Review: A Guide for Manuscript Reviewers"
- "Do you have any real or apparent conflicts of interest?
Is the work too close to your own? Sometimes a potential reviewer is presented with a very awkward problem when he/she is asked to review a paper that is very close to his/her own work. As will be discussed below, manuscripts under review are considered confidential documents. By agreeing to review a manuscript, the reviewer assumes an obligation to keep the data in confidence and not to use it for his/her own benefit. This can raise a problem when a reviewer receives a request to review a paper which reports experiments that overlap with studies that the reviewer is already performing, planning to perform, or preparing for publication. - The potential reviewer should not review this paper: doing so presents a “no-win” situation even if the reviewer acts with the utmost integrity. If the paper is good and the reviewer were to review it rapidly and recommend acceptance, he/she might well compromise his/her ability to publish his/her own work – this knowledge creates an immediate, significant conflict of interest. On the other hand, if the paper proves to be flawed and the reviewer (with all integrity) were to recommend extensive revisions or rejection, the perception of misconduct could arise in the editor’s mind when the reviewer’s own studies were submitted or published.
- The reviewer is therefore in an unfortunate situation in which even totally ethical actions could produce unpleasant outcomes, and should take immediate steps to minimize the potential for adverse effects. The potential reviewer should contact the editor immediately, inform the editor of the problem, and decline to review the paper. If at all possible, this should be done when the reviewer has seen only the title and abstract; the reviewer should make every effort to ensure that he/she does not receive the complete paper. The procedures now being used for electronic reviews are helpful in this regard – but there is still a possibility that a colleague or an editor will give a potential reviewer a complete manuscript along with a request for a review, in which case an immediate discussion of the problem and return of the manuscript are essential." (Source: U.S. Department of Health and Human Services (HHS) Office of Research Integrity (ORI) "Ethics of Peer Review: A Guide for Manuscript Reviewers" Sara Rockwell, Ph.D. Departments of Therapeutic Radiology and Pharmacology, and Office of Scientific Affairs, Yale University School of Medicine, PO Box 208040, New Haven, CT 06510-8040. p. 5)
"COPE Ethical Guidelines for Peer Reviewers - English"
- "Confidentiality: Respect the confidentiality of the peer review process and refrain from using information obtained during the peer review process for your own or another’s advantage, or to disadvantage or discredit others (e.g. see COPE Case 14-06: Possible breach of reviewer confidentiality). Do not involve anyone else in the review of a manuscript (including early career researchers you are mentoring), without first obtaining permission from the journal (e.g. see COPE Case 11-29: Reviewer asks trainee to review manuscript). The names of any individuals who have helped with the review should be included so that they are associated with the manuscript in the journal’s records and can also receive due recognition for their efforts." (Source: COPE Council. Ethical guidelines for peer reviewers. September 2017 Version 2 Published September 2017. www.publicationethics.org)
- "How do you handle the paper?
Manuscripts under review are confidential documents, and should be treated as such. They contain unpublished data and ideas that must be kept confidential. You cannot share the paper or its contents with your colleagues. Manuscripts that you are reviewing should be kept in a secure place, where they will not be readily accessible to the curious or unscrupulous. Moreover, you cannot use the information in the paper in your own research or cite it in your own publications. This can raise serious ethical issues if the work provides insights or data that could benefit your own thinking and studies. An especially difficult situation is that in which you receive a manuscript which has serious flaws, causing that you recommend rejection or major revisions that will require time and new experiments to address, but also contains an element that you find exciting and would like to explore yourself. Such cases can become ethical quagmires." (Source: ibid.)
"Peer-Review Fraud — Hacking the Scientific Publication Process"
- "How is it possible to fake peer review? Moon, who studies medicinal plants, had set up a simple procedure. He gave journals recommendations for peer reviewers for his manuscripts, providing them with names and e-mail addresses. But these addresses were ones he created, so the requests to review went directly to him or his colleagues. Not surprisingly, the editor would be sent favorable reviews — sometimes within hours after the reviewing requests had been sent out. The fallout from Moon's confession: 28 articles in various journals published by Informa were retracted, and one editor resigned. 3
- (...)
The problem is the perverse incentive systems in scientific publishing. As long as authors are (mostly) rewarded for publishing many articles and editors are (mostly) rewarded for publishing them rapidly, new ways of gaming the traditional publication models will be invented more quickly than new control measures can be put in place." (Source: "Peer-Review Fraud — Hacking the Scientific Publication Process" Charlotte J. Haug, M.D., Ph.D. The New England Journal of Medicine 2015; 373:2393-2395. December 17, 2015. DOI: 10.1056/NEJMp1512330)
- "Interview with Dr. Charlotte Haug on the growing number of article retractions attributable to peer-review fraud." (Supplement to the N Engl J Med 2015; 373:2393-2395)
Peer Review (continued)
"When reviewing goes wrong: the ugly side of peer review"
"Uncovering new peer review problems – this time at The BMJ"
"Increasing Transparency Around Peer Review and Research"
"The changing forms and expectations of peer review"
"The ability of different peer review procedures to flag problematic publications"
"A reviewer stole a manuscript and published it himself. But you wouldn’t know it from this retraction notice."
"Ethical Reviewers are Essential for Scholarly Journals for Timely Processing of Submissions and Avoiding Retractions"
"Volunteers Fight Bad Science"
"When reviewing goes wrong: the ugly side of peer review"
- "The research community has become hyper competitive, which stems in large part from the intense pressure on publication and the associated impact on evaluation and progression. In some countries, this pressure comes from institutes or funding agencies; in others directly from government. Another significant contributor to the issue is the increasing difficulty of finding suitable reviewers with the requisite expertise, time and willingness." (Source: "When reviewing goes wrong: the ugly side of peer review" Christopher Tancock. Elsevier Connect, Editors' Update March 23, 2018)
"Uncovering new peer review problems – this time at The BMJ"
- ‘Multiple breakdowns in the process’
“This looks really bad,” said Melissa S. Anderson, PhD, a professor of higher education at the University of Minnesota who previously co-chaired the World Conference on Research Integrity. “Why would they want to risk being accused of conflict of interest when [the BMJ peer review process] is open? That amazes me. I don’t know why you’d do that.”
Anderson said that even if these reviewers provided an accurate assessment of the science, the fact that they are close collaborators of the authors casts significant doubt on their conclusions. “If it looks bad, it’s a conflict of interest,” she said. “That actually is becoming the standard for how you determine if something is a conflict of interest. Even the appearance of a conflict constitutes an actual conflict.”
(Source: Committee on Publication Ethics (COPE). "Uncovering new peer review problems – this time at The BMJ")
- "The Committee on Publication Ethics (COPE) has become aware of systematic, inappropriate attempts to manipulate the peer review processes of several journals across different publishers. These manipulations appear to have been orchestrated by a number of third party agencies offering services to authors. This statement is issued on behalf of COPE after consultation with a variety of publishers to underscore the seriousness with which we take these issues and our determination to address them.
While there are a number of well-established reputable agencies offering manuscript-preparation services to authors, investigations at several journals suggests that some agencies are selling services, ranging from authorship of pre-written manuscripts to providing fabricated contact details for peer reviewers during the submission process and then supplying reviews from these fabricated addresses. Some of these peer reviewer accounts have the names of seemingly real researchers but with email addresses that differ from those from their institutions or associated with their previous publications, others appear to be completely fictitious." (Source: "COPE statement on inappropriate manipulation of peer review processes" 19/12/2014)
- "What all these cases had in common was that researchers exploited vulnerabilities in the publishers' computerized systems to dupe editors into accepting manuscripts, often by doing their own reviews. The cases involved publishing behemoths Elsevier, Springer, Taylor & Francis, SAGE and Wiley, as well as Informa, and they exploited security flaws that — in at least one of the systems — could make researchers vulnerable to even more serious identity theft. “For a piece of software that's used by hundreds of thousands of academics worldwide, it really is appalling,” says Mark Dingemanse, a linguist at the Max Planck Institute for Psycholinguistics in Nijmegen, the Netherlands, who has used some of these programs to publish and review papers.
(...)
In what Lindsay calls the worst case that he has seen, an author suggested a reviewer who shared her first name but not her surname. Some investigation revealed that the surname was the author's maiden name — she was recommending that she review her own paper. “I don't think she is going to submit anything to us again,” says Lindsay." (Source: "Publishing: The peer-review scam" Cat Ferguson, Adam Marcus& Ivan Oransky. Nature 515, 480–482 (27 November 2014) doi:10.1038/515480a)
- "Imperfect System
Ivan Oransky, a health journalism professor at New York University and co-founder of Retraction Watch, a website that tracks errors in science journals, believes that, while the error was unfortunate for the authors, part of the issue lies in the amount of faith the public — and journalists — put in the peer-review process.
Science is done by human beings," said Oransky. "When we start to think about peer review as a magical process that, somehow you put in something that may have some issues, but somehow the peer reviewers find all the problems, and then we should trust everything that's peer reviewed, we're setting ourselves up for failure.
He still believes in the process, but says it's not the catch-all that scientists and journalists believe it is.
We need to stop thinking… that just because something is peer-reviewed means that it's a Good Housekeeping seal of approval," Oransky said." (Source: "Correction to climate change study highlights flaws in peer-review process" Nicole Mortillaro, CBC News · Posted: Nov 16, 2018 4:00 AM ET, Last Updated: November 16)
"Increasing Transparency Around Peer Review and Research"
- "Obstetrics & Gynecology employs a single-blind peer-review process, in which the authors do not know the identity of the reviewers unless the reviewer voluntarily self-identifies. We will continue to do so. However, starting in July 2018 and phased in over several months, we will lift the curtain of the peer-review process for accepted articles and implement what is termed “transparent peer review.” Starting with this month's issue, we will publish the dates an article is submitted, revised, and accepted, increasing transparency. Then, later this year, readers will start to see the peer reviewers' and editor's comments to the author, along with the authors' responses to these comments, published as supplemental digital content to the online version of each peer-reviewed article. Authors can opt out of having their responses to these comments published if they wish to do so. Reviewer invitations will inform them that their anonymous reviews will be published online; invited reviewers can opt out by declining the invitation. The iterative communication from the editor to the authors (and their responses if they opt in) will also be published so that interested readers can see the process of moving a manuscript from submission to publication. Even if the authors decline to have their responses published, the reviewer and editor comments will be posted online." (Source: Editorial: "Increasing Transparency Around Peer Review and Research" Chescheir, Nancy C., MD. Obstetrics & Gynecology: July 2018 - Volume 132 - Issue 1 - p 1–2. doi: 10.1097/AOG.0000000000002712.)
"The changing forms and expectations of peer review"
- "Background
Quality and integrity in science
Recently, there has been heated debate on the quality, credibility and integrity of scientific literature. Due to a perceived increase in scientific fraud and irreproducible research, some claim the publication system, or even science in general, to be in crisis [9, 25]. This rising concern has become obvious in the media, in policy initiatives, as well as in scientific literature. Concerned scientists as well as policymakers increasingly express their worry about data manipulation, plagiarism, or questionable research practices that affect the functioning of science [56].
(...)
We will demonstrate that these new expectations are not always entirely compatible with one another and hence lead to tensions in the current academic debate about what peer review can and should do. Underlying this debate, we note a growing expectation that the scientific literature will serve as a database of established knowledge, rather than as a collection of research reports, pointing to more fundamental disagreement about the nature of scientific knowledge. At least some of the expectations of peer review are not just about the practicalities of ‘how to make it work better’; many also expect the process to address the functions of the publication system and even what it means to publish an account of a research project." (Source: "The changing forms and expectations of peer review" S. P. J. M. ( Serge) Horbach and W. ( Willem) Halffman. Research Integrity and Peer Review (2018) 3:8. https://doi.org/10.1186/s41073-018-0051-5. View/Download pdf here.)
"The ability of different peer review procedures to flag problematic publications"
- "Abstract
There is a mounting worry about erroneous and outright fraudulent research that gets published in the scientific literature. Although peer review’s ability to filter out such publications is contentious, several peer review innovations attempt to do just that. However, there is very little systematic evidence documenting the ability of different review procedures to flag problematic publications. In this article, we use survey data on peer review in a wide range of journals to compare the retraction rates of specific review procedures, using the Retraction Watch database."
"Conclusion
In spite of various calls for more research on the effectiveness of various peer review procedures, actual evidence is rare. This study addresses precisely this knowledge gap. Our analyses reveal major differences between various ways of organising peer review and the number of retracted journal articles going through these review procedures. Thereby, they provide an indication of the effectiveness of various peer review models in detecting erroneous or fraudulent research. Even though hard causal connections cannot be made, our data suggest that some review procedures are significantly more effective in preventing retractions. In particular, author blinding seems more effective than reviewer blinding; involving the wider community in review seems beneficial; using digital tools to assist review is related to fewer retractions, as is constraining interaction between authors and reviewers, and using pre-submission review procedures such as registered reports. In addition, our data suggest differences in the effectiveness of various review procedures between scientific disciplines, as well as between specific reasons for retraction. Thereby we present a systematic comparison of review procedures’ effectiveness in detecting problematic research publications." (Source: "The ability of different peer review procedures to flag problematic publications" Horbach, S.P.J.M. & Halffman, W. Scientometrics (2018) pp 1–35. https://doi.org/10.1007/s11192-018-2969-2. Received 02 October 2018, First Online 29 November 2018. View/Download pdf here.)
"A reviewer stole a manuscript and published it himself. But you wouldn’t know it from this retraction notice."
- "In a guest editorial for the Journal of Korean Medical Science — which wasn’t involved in the heist — Mehregan began by noting the toll that protracted peer review can take on authors:
- "When the review comments are not returned back in due time, there is a possibility that the invited reviewer does not submit the evaluation results in a timely manner intentionally so that he could have sufficient time to publish that original research in another journal as if it is his own work." (Source: "A reviewer stole a manuscript and published it himself, but you wouldn’t know it from this retraction notice.")
"Ethical Reviewers are Essential for Scholarly Journals for Timely Processing of Submissions and Avoiding Retractions"
- "Today there are many peer-reviewed established international journals where scholars can submit their research works. There are a variety of factors that affect the authors' choices of the target journals.1 In addition to the reputation of the journal in some specific research areas and its impact factor, the short reviewing process time is another important parameter for scholars to choose the right journal to submit their manuscripts. However, unfortunately a large number of prestigious journals are not concerned about the duration of the peer review. A long review process may not be an issue for the journals, but it causes the authors some troubles. We are living in a century of technology and time plays an important role in our lives. When the evaluation comments get back after a long time, there could be a possibility that the idea of the research is not as novel as the time when the work has been submitted. There is also a more important issue which should be noticed. When the review comments are not returned back in due time, there is a possibility that the invited reviewer does not submit the evaluation results in a timely manner intentionally so that he could have sufficient time to publish that original research in another journal as if it is his own work. This is exactly what happened to one of our recent works published in Environmental Science and Pollution Research journal.2 The reviewing process of the submitted paper took about nine months. After the publication of the original research, one day I accidentally noticed that the same work has been published into another international journal by other authors. I contacted the journal we published in and was informed that the Indian corresponding author of the plagiarized paper (https://doi.org/10.1080/15567036.2017.1407844) was the reviewer of our manuscript. Fortunately, the plagiarized paper is now retracted, however—despite all the clear evidences regarding this blatant plagiarism—the investigation process by the journal to withdraw the offending item took about 5 months and this fraudulent article has been cited 17 times (8 self-citations, 3 citations by the authors from the same affiliation and 6 ones by the authors with the same nationality) during this time. There is a series of flow charts provided by Committee on Publication Ethics (COPE) to outline the steps which should be taken by journals when they face plagiarism.3 According to the retraction guideline, plagiarized publications should be withdrawn as soon as possible in order to minimize the number of citations to the erroneous work.4 As well as, in the case of clear plagiarism, the editor has to inform the author's institution and also readers and victims(s) of the offending article to avoid these inauthentic citations.5" (Source: "Ethical Reviewers are Essential for Scholarly Journals for Timely Processing of Submissions and Avoiding Retractions" Mina Mehregan. J Korean Med Sci. 2019 Feb 04;34(5):e41. English. Published online Jan 28, 2019. https://doi.org/10.3346/jkms.2019.34.e41) [Note: Also see: "A reviewer stole a manuscript and published it himself. But you wouldn’t know it from this retraction notice." Adam Marcus. Retraction Watch February 14, 2019]
"Volunteers Fight Bad Science"
- "James Heathers is a postdoctoral researcher at Northeastern University, who looks for mistakes for fun. He speaks to NPR's Lulu Garcia-Navarro speaks [sic] about errors published in scientific papers." [Link to complete NPR audio interview provided below.]
(...)
HEATHERS: Well, if that sounds ominous, it probably should. Bad medical studies can tip larger results one way or another. So imagine we have six studies, and one of them is problematic for some reason. And when we add up the results of all the six studies and we do a meta-analysis, that one, individual, bad study changes the outcome of the meta-analysis. That can influence how governments buy drugs, how frontline hospital care is conducted and what is considered to be standard care as evidence-based or not so evidence-based. So at its absolute worst, there's very definitely problems like that that exist right now. I'm afraid I can't really speculate any further than that." (Source: "Volunteers Fight Bad Science" James Heathers. NPR: Science. Heard on Weekend Edition Sunday. February 17, 20198:12 AM ET)
Peer Review (continued)
"Paper used to support WHO guidelines on preventing infections “has no scientific validity”"
"Regenerating the field of cardiovascular cell therapy"
"Just another week in real-world science: Butler, Pentoney, and Bong (2017)."
Peer Review (continued)
ReimagineReview
"Paper used to support WHO guidelines on preventing infections “has no scientific validity”"
- "A surgery journal retracted a 2014 paper last month after discovering that the study has “no scientific validity.”"
(...)
The 2014 study in The American Journal of Surgery was one among 11 cited in a World Health Organization analysis used to support its 2016 guidelines recommending supplemental oxygen to prevent surgical infections in adults. (A 2012 paper from the authors was also part of that analysis.)" (Source: "Paper used to support WHO guidelines on preventing infections “has no scientific validity”" Victoria Stern. Retraction Watch. March 26, 2018)
"Regenerating the field of cardiovascular cell therapy"
- "Abstract
The retraction of >30 falsified studies by Anversa et al. has had a disheartening impact on the cardiac cell therapeutics field. The premise of heart muscle regeneration by the transdifferentiation of bone marrow cells or putative adult resident cardiac progenitors has been largely disproven. Over the past 18 years, a generation of physicians and scientists has lost years chasing these studies, and patients have been placed at risk with little scientific grounding. Funding agencies invested hundreds of millions of dollars in irreproducible work, and both academic institutions and the scientific community ignored troubling signals over a decade of questionable work. Our collective retrospective analysis identifies preventable problems at the level of the editorial and peer-review process, funding agencies and academic institutions. This Perspective provides a chronology of the forces that led to this scientific debacle, integrating direct knowledge of the process. We suggest a science-driven path forward that includes multiple novel approaches to the problem of heart muscle regeneration." (Source: "Regenerating the field of cardiovascular cell therapy" Kenneth R. Chien, Jonas Frisén, Regina Fritsche-Danielson, Douglas A. Melton, Charles E. Murry & Irving L. Weissman. Nature Biotechnology (2019). Published: 18 February 2019)
"Just another week in real-world science: Butler, Pentoney, and Bong (2017)."
- "There seems to be a real problem with academic editors, especially those at the journals of certain publishers, being reluctant, unwilling, or unable to take action on even the simplest problems without the approval of the publisher, whose evaluation of the situation may be based as much on the need to save face as to correct the scientific record.
A final anecdote: One of us (Nick) has been told of a case where the editor would like to retract at least two fraudulent articles but is waiting for the publisher (not Elsevier, in that case) to determine whether the damage to their reputation caused by retracting would be greater than that caused by not retracting. Is this really the kind of consideration to which we want the scientific literature held hostage?" (Source: "Just another week in real-world science: Butler, Pentoney, and Bong (2017)." Nick Brown's blog, "Random" doesn't even come close. Nick Brown and Stuart Ritchie. 19 February 2019)
Peer Review (continued)
ReimagineReview
- "ReimagineReview is a registry of platforms and experiments innovating around peer review.
Peer review is deeply embedded into the scientific process, and it serves multiple purposes: giving and receiving feedback, error detection and correction, and filtering and curation. Yet, there’s little evidence that it functions optimally.
Projects listed in ReimagineReview seek to improve or extend this process in inventive ways, making it more open, unbiased, rapid, robust, or accessible." (Source: "ReimagineReview is a project of ASAPbio funded by CZI and developed in partnership with Wellcome and the Howard Hughes Medical Institute." https://reimaginereview.asapbio.org/) - "Peer-review experiments tracked in online repository"
"ReimagineReview records trials that are probing the pros and cons of different approaches to review." (Source: "Peer-review experiments tracked in online repository" Richard Van Noorden. Nature 04 MARCH 2019. doi: 10.1038/d41586-019-00777-8)
Plagiarism
As defined by the World Association of Medical Editors (WAME)
"Idea-plagiarism and precedence: ethics in competitive research"
"Credit and Priority in Scientific Discovery: A Scientist’s Perspective"
"Chapter 2: Plagiarism and Academic Misconduct" from False Feathers: A Perspective on Academic Plagiarism
"Plagiarism detectors are a crutch, and a problem"
"Academics and editors need to stop pretending that software always catches recycled text and start reading more carefully, says Debora Weber-Wulff."
"Plagiarism by Academics: More Complex than it Seems"
"Definition
Plagiarism resembles pornography, at least to the extent that people believe that they know it when they see it, but seldom take the trouble to construct, or to seek out a definition of the term. Dictionary entries include the following:
"Analyzing the definitions and usage, I identify several elements of the notion of plagiarism:
Plagiarism (continued)
U.S. Department of Health and Human Services (HHS) Office of Research Integrity (ORI)
Plagiarism (continued)
Plagiarism in Combination With Falsification and/or Fabrication
"Cases of Plagiarism Handled by the United States Office of Research Integrity 1992–2005"
Plagiarism (continued)
Definitions
Plagiarism (continued)
Plagiarism (continued)
"Plagiarism: Why is it such a big issue for medical writers?"
"Responding to Possible Plagiarism"
"Documenting reactions from authors and journal editors to plagiarism may help others address the problem."
"Authorship: The Coin of the Realm, The Source of Complaints"
"Even potential participants of a research integrity conference commit plagiarism, organizers learn"
NORWEGIAN MINISTRY OF EDUCATION AND RESEARCH
Consultation on revision of the Research Ethics Act (Høring om revisjon av forskningsetikkloven)
"Consultation paper – Research Ethics in Norway" (p. 36)
Norway's Research Ethics Act
"Dear peer reviewer, you stole my paper: An author’s worst nightmare"
"Chem journal yanks paper because authors had stolen it as peer reviewers"
"Peer reviewer stole article and published it as his own"
"knowingly, intentionally, or recklessly [appropriated] the ideas, processes, results or words”
"Prominent US critic of cancer screening committed plagiarism, investigation finds"
Plagiarism (continued)
"Background: Plagiarism is more than just copying someone else's text"
"Bakgrunn: Plagiat er mer enn bare å kopiere en annens tekst"
Plagiarism (continued)
Committee on Publication Ethics (COPE)
"What to do if you suspect plagiarism"
Plagiarism (continued)
"A colleague included plagiarized material in your grant proposal. Are you liable?"
Plagiarism (continued)
Public exposure of plagiarists
"Iain Chalmers describes a case of scientific misconduct in which an author plagiarised both text and data on two separate instances.1 Although he took the proper steps after detecting the plagiarism, Chalmers seems to have been unsuccessful in resolving the matter. This and other similar failures of our scientific system of justice leads me to support his recommendations for dealing with plagiarism, particularly his call to publicly expose those who have been found guilty of misconduct.
Public exposure of plagiarists, and the consequent embarrassment and ostracism that these offenders should experience, not only satisfies our intrinsic need for social justice but can also serve as a deterrent. Unfortunately, many scientific journals, professional organisations, and academic institutions lack the necessary resources and, apparently in some cases, even the will to investigate misconduct allegations." (Source: "Ethical writing should be taught" Miguel Roig. BMJ 2006;333:596 doi: https://doi.org/10.1136/bmj.38946.501215.68 Published 14 September 2006. ) [Note: Citation 1: Chalmers I. Role of systematic reviews in detecting plagiarism: case of Asim Kurjak. BMJ 2006;333: 594–6. View/download PDF here. "Naming and shaming" and "Action" excerpts are included below.]
"Naming and shaming
Although this is but one case study [case of Asim Kurjak], it prompts me to make some general suggestions for detecting and reducing plagiarism. Firstly, journals and institutions should heed Nature’s editorial call 1 to take allegations of plagiarism more seriously than they seem to at present. Secondly, as has been suggested by Tom Jefferson and colleagues,9 10 journals should use systematic reviews for editorial peer review, as well as considering using software designed to detect plagiarism. Lastly, to reduce the numbers of new as well as recurrent plagiarists, journals, institutions, and professional associations need to expose very publicly those found guilty of this form of scientific misconduct." (Source : Analysis And Comment, Professional regulation, "Role of systematic reviews in detecting plagiarism: case of Asim Kurjak" Iain Chalmers. BMJ 2006;333:594. p. 595-596. (Published 14 September 2006) doi: https://doi.org/10.1136/bmj.38968.611296.F7. View/download PDF here.) [Note: Citation 1: "Complacency about misconduct" Editorial, Nature volume 427, page 1. 01 January 2004)
"Action
More than 15 years have passed since I detected Professor Kurjak’s plagiarism and reported it to the relevant authorities. Yet, as the Norwegian case shows, he seems to believe that he can continue to act with impunity in this respect. This continued scientific misconduct raises serious doubt about the extent to which his publications are trustworthy accounts of his own work. Despite this record of scientific misconduct, Professor Kurjak is clearly regarded with great respect, not only within Croatia but also by many obstetricians elsewhere. The case I have described is an illustration of a more widespread problem. What could or should a whistleblower like me expect from institutions and journals when plagiarism is reported to them. I can illustrate some general principles by itemising my current expectations in this case:
"Plagiarism and punishment"
"The Kurjak plagiarism case: the vicious circle of academic corruption
The cloud now hanging over Croatia's research community (1) is dark indeed. The Court of Honour of Zagreb University’s Medical School has dismissed proven allegations of Professor Kurjak's misconduct (2) on the
grounds that his misconduct had occurred long ago, that the culprit had already apologised, and that he recently retired (3). The decision of the Court of Honour did not refer to the fact that the national Committee for Ethics in Science and Higher Education of the Republic of Croatia (4) had confirmed the allegations made in Chalmers’ article published in the BMJ last year (2), and had found additional examples of Kurjak’s unethical practices after evaluating his whole publication opus (5). Nor did the Medical School’s Court of Honour mention that, in July this year, the Ministry of Science, Education and Sports suspended the funding of Kurjak's research grant (6).
(...)
The Economist recently suggested that “the concept of conflict of interest is little understood” in Croatia (12). The actions of the University of Zagreb and the Medical School suggest that the academic community in Zagreb believes that its autonomy provides it with immunity from its responsibility to the public. Invited to comment on Kurjak’s misconduct during a TV show, University Rector Bjelis stated that plagiarism was a benign problem and that the University had more important problems to address. Institutional failure to deal with research misconduct occurs in other academic communities (13), but a worrying aspect of the failures described above is their possible impact on the prospects for Croatia’s accession to the European Union (14). (Source: "Plagiarism and punishment" "The Kurjak plagiarism case: the vicious circle of academic corruption" Matko Marusic, Editor in Chief, Croatian Medical Journal, Zagreb, Croatia. BMJ 2007; 335:0 doi: https://doi.org/10.1136/bmj.39392.602523.47 (Published 08 November 2007))
"Croatia is let down"
"In November 2006 the editors of the Croatian Medical Journal asked the Committee on Publication Ethics (COPE) to help it in dealing with allegations against Asim Kurjak of duplicate or redundant publication, or both. These were quite separate from the previous allegations of plagiarism by this author.
An investigation by COPE concluded in February 2007, in regard to the papers submitted to it by the journal, that there is strong evidence that Kurjak (or his co-authors) committed publication misconduct on at least three occasions in relation to papers submitted to the Croatian Medical Journal. In two cases, papers co-authored by Kurjak were submitted to the journal after similar papers had been accepted by another journal, and in one case the material published in the Croatian Medical Journal seems to have been inappropriately republished in another journal.
COPE advised the editors of the Croatian Medical Journal to send its report to the University of Zagreb, requesting that it conduct an inquiry into the allegations.
One problem that has beset COPE since it was founded in 1997 has been the apparent reluctance of some institutions to take seriously complaints made to them by editors about probable publication misconduct by their staff or employees. The University of Zagreb now seems to be one of them. COPE is appalled by Marusic’s revelation (previous letter)1 that the report was swept under the carpet by those entrusted with maintaining the integrity of research at the university’s medical school.
Far from protecting the name of the university and its medical school, this action only serves to diminish their reputations and to cast doubt on the undoubted body of reliable and honest research carried out there.
COPE has written to the dean of the medical school, the rector of the university, and the Croatian minister of health, Professor Primorac, expressing its dismay. Let us hope that the minister directs that the court of honour at Zagreb University be reconvened to consider the complaint from the Croatian Medical Journal." (Source: "Croatia is let down" "The Kurjak plagiarism case" Harvey Marcovitch, Past Chairman of COPE. BMJ. 2008 Jan 26; 336(7637): 174. doi: 10.1136/bmj.39450.661528.3A.)
Plagiarism (continued)
Plagiarism (continued)
"Researchers accuse German authority of plagiarism in glyphosate review"
New report casts further doubt on EU’s glyphosate assessments.
"Journal to retract article from 2000 that plagiarized one from 1984"
"Analysis of retractions in Indian science"
Plagiarism (continued)
Plagiarism Euphemisms
"The Euphemism Parade"
"Why can’t journals just come out and say “plagiarism”?
Science is supposed to be about cold hard truths, about tested hypotheses and convincingly small p values. So why are journal editors so afraid of the p word?
Take these examples of the lengths editors have gone to in order to avoid using the word “plagiarism” in retraction notices:
"Et Plagieringseventyr" (A Plagiarism Carol), a Norwegian video parody of Charles Dickens' novella A Christmas Carol with English subtitles (Source: University of Bergen (UiB) Published on May 27, 2010. YouTube: https://youtu.be/Mwbw9KF-ACY)
As defined by the World Association of Medical Editors (WAME)
- "Plagiarism is the use of others' published and unpublished ideas or words (or other intellectual property) without attribution or permission, and presenting them as new and original rather than derived from an existing source. The intent and effect of plagiarism is to mislead the reader as to the contributions of the plagiarizer. This applies whether the ideas or words are taken from abstracts, research grant applications, Institutional Review Board applications, or unpublished or published manuscripts in any publication format (print or electronic).
- Plagiarism is scientific misconduct and should be addressed as such (see prior section).
- Self-plagiarism refers to the practice of an author using portions of their previous writings on the same topic in another of their publications, without specifically citing it formally in quotes. This practice is widespread and sometimes unintentional, as there are only so many ways to say the same thing on many occasions, particularly when writing the Methods section of an article. Although this usually violates the copyright that has been assigned to the publisher, there is no consensus as to whether this is a form of scientific misconduct, or how many of one's own words one can use before it is truly "plagiarism." Probably for this reason self-plagiarism is not regarded in the same light as plagiarism of the ideas and words of other individuals. If journals have developed a policy on this matter, it should be clearly stated for authors." (Source: "Recommendations on Publication Ethics Policies for Medical Journals" The Publication Ethics Committee, World Association of Medical Editors (WAME), Accessed 25 March 2020)
"Idea-plagiarism and precedence: ethics in competitive research"
- However, plagiarism of words is not the only form that plagiarism can take in research. Scientists invest thought and ideas while pursuing research, whether that research be confirmatory, incremental, or path-breaking. Many researchers believe that new ideas lie at the heart of research that is subsequently classified as novel. Some new idea would be necessary for incremental research, and maybe an out-of-the-box idea for research that can be described as path-breaking. As active researchers are pushed to move from confirmatory research to incremental or path-breaking research, the role of ideas becomes more important than the role of words in our research papers.
(...)
Idea plagiarism, with a clever manipulation of words, can result in an undeserving person being honoured and thereby getting unjustified societal credibility, a credibility that results in society accepting advice and suggestions from sources who may disseminate false information." (Source: "Idea-plagiarism and precedence: ethics in competitive research" Praveen Chaddah. IndiaBioscience, Posted on Aug 10, 2018 in ADVICE and ETHICS. "IndiaBioscience is majorly funded by Department of Biotechnology (DBT), Government of India.")
"Credit and Priority in Scientific Discovery: A Scientist’s Perspective"
- "It is difficult to define a precise border between healthy and productive credit-seeking behaviors and those that are excessive or pathologic. In its most extreme varieties, pathologic interest in credit and recognition undermines the primary commitment to the truth. How might this occur? Excessive desire for credit may lead susceptible scientists to promote or tolerate sloppiness and errors, or engage in one of the hallmarks of scientific misconduct—plagiarism, falsification, or fabrication—even if such “credit” is in the end short-lived. There is of course no moral justification for credit-seeking that leads scientists to abandon their fealty to the truth. But history shows that pathologic desire for credit drives a small minority of scientists to this deeply unfortunate outcome. The scientific community must take seriously behaviors that violate the norms of science via exaggerated pursuit of credit, and devise and deploy approaches to reduce these unfortunate outcomes." (Source: "Credit and Priority in Scientific Discovery: A Scientist’s Perspective" Jeffrey S. Flier. Perspectives in Biology and Medicine, Ahead of Print, (Article). Published by Johns Hopkins University Press DOI: https://doi.org/10.1353/pbm.0.0018. p. 18)
"Academic Institutions Must Assume Greater Responsibility for the Allocation of Credit"
"Credit for discovery is the “currency of the realm”, and the realm in which the currency operates is the community of academic institutions. Just as governments issuing financial currency are responsible for limiting counterfeit varieties, so must the academy assume greater responsibility for limiting counterfeit academic credit." (Source: Ibid., p. 19)
"Chapter 2: Plagiarism and Academic Misconduct" from False Feathers: A Perspective on Academic Plagiarism
- "Chapter 2
Plagiarism and Academic Misconduct
"If one wants plagiarism and academic misconduct to be addressed fairly and consistently, there must be good definitions available that are more or less universally agreed upon. This is where the trouble begins, as there are numerous definitions in English that focus on different aspects of the problem. In Germany, however, there is currently no legal definition of plagiarism, in particular because the responsibility for universities lies with the power of the states, not with the federal government. The states, in turn, tend to delegate responsibility for such topics to the universities. Each university will, if attempting to define it at all, have its own definition of the term. So in the absence of a legal definition, any plagiarism cases that end up in court force the judges to look at prior law on the topic instead of being able to refer to an codified definition.
This chapter will be concerned with presenting and discussing some of the various definitions for plagiarism and academic misconduct, as well as examining the various types of plagiarism. A section on the magnitude of the problem and the reasons given by students for cheating is followed by a discussion of the reasons that plagiarism is a problem.
2.1 Definition of Plagiarism
The word plagiarism is derived from the Latin word plagiarius, meaning someone who kidnaps the child or slave of another. A Latin poet, Marcus Valerius Martialis, used this term in Epigrams 1, 52 to describe a fellow poet, Quintianus, who published Martial’s poems as his own. Martial felt that his poems were the children of his mind, and that they were being stolen. The term, however, only began being commonly used to describe what is sometimes also called literary theft around 1600." (Source: "Chapter 2: Plagiarism and Academic Misconduct" from False Feathers: A Perspective on Academic Plagiarism Debora Weber-Wulff. Springer, Berlin, Heidelberg 2014. p. 3. Print ISBN 978-3-642-39960-2 Online ISBN 978-3-642-39961-9 DOI https://doi.org/10.1007/978-3-642-39961-9_2)
"Plagiarism detectors are a crutch, and a problem"
"Academics and editors need to stop pretending that software always catches recycled text and start reading more carefully, says Debora Weber-Wulff."
- "This year, abstracts submitted to the World Conference on Research Integrity were analysed by software, with a text-overlap threshold set at 30%. And, indeed, 38 out of 449 submitted abstracts registered above this level. After investigating, 15 were considered to be plagiarism and 23 contained text from the author’s previously published research. Most of the abstracts were rejected; in some of the instances in which authors had recycled their own text, the abstracts were demoted to posters. This amount of plagiarism and duplication is shocking, especially for a conference on academic integrity; it is also probably an underestimate.
Software cannot determine plagiarism; it can only point to some cases of matching text. The systems can be useful for flagging up problems, but not for discriminating between originality and plagiarism. That decision must be taken by a person. The most important method for finding plagiarism is reading a text and studying the references for inconsistencies. A spot check with an Internet search engine, using three to five words from a paragraph or a particularly nice turn of phrase can uncover copyists. Searching for a reference that looks odd might turn up a source that mangled the reference in the same manner. Only if a text is somehow off, and online searching does not help, should software systems be consulted. In those cases, it’s best to use two or three systems, and to read the reports, not take the numbers at face value.
Academic integrity is a social problem; due diligence cannot be left to unknown algorithms. Keeping science honest depends on scientists willing to work hard to protect the literature." (Source: "Plagiarism detectors are a crutch, and a problem" Debora Weber-Wulff. Nature 567, 435 (2019) doi: 10.1038/d41586-019-00893-5. 27 MARCH 2019)
"Plagiarism by Academics: More Complex than it Seems"
"Definition
Plagiarism resembles pornography, at least to the extent that people believe that they know it when they see it, but seldom take the trouble to construct, or to seek out a definition of the term. Dictionary entries include the following:
- To take and use another person's (thoughts, writings, inventions ...) as one's own (Oxford);
- To steal or purloin from the writings of another; to appropriate without due acknowledgement (the ideas or expressions of another) (Merriam-Webster);
- To appropriate or imitate another's ideas and manner of expressing them, as in art, literature, etc., to be passed off as one's own (Macquarie);
- To copy and pass off (the expression of ideas or words of another) as one's own; to use (another's work) without crediting the source (Merriam-Webster Dictionary of Law).
"Analyzing the definitions and usage, I identify several elements of the notion of plagiarism:
- publication: the presentation of another person's material, work, or idea. A pre-condition for plagiarism is that the new work is made available to others; personal notes are not at issue;
- content: the presentation of another person's material, work, or idea. A pre-condition for plagiarism is that some part of the new work is derived from someone else's prior or contemporaneous work;
- appropriation: the presentation of another person's material, work, or idea as one's own. A pre-condition for plagiarism is that the claim of originality of contribution is either explicit or implied by the manner of presentation; or the presentation may be such that the reader is reasonably likely to infer the work to be an original contribution; and
- lack of credit given: the presentation of another person's material, work, or idea as his or her own, without appropriate attribution. A pre-condition for plagiarism is that the reader is not made aware of the identity of the originator, nor of the location of the original contribution.
Plagiarism (continued)
U.S. Department of Health and Human Services (HHS) Office of Research Integrity (ORI)
- ORI Policy on Plagiarism
"As a general working definition, ORI considers plagiarism to include both the theft or misappropriation of intellectual property and the substantial unattributed textual copying of another's work. It does not include authorship or credit disputes.
The theft or misappropriation of intellectual property includes the unauthorized use of ideas or unique methods obtained by a privileged communication, such as a grant or manuscript review." (Source: U.S. Department of Health and Human Services (HHS) Office of Research Integrity (ORI): "ORI Policy on Plagiarism")
- "Plagiarism Taking over the ideas, methods, or written words of another, without acknowledgment and with the intention that they be taken as the work of the deceiver. American Association of University Professors (September/October,1989)
As the above quotation shows, plagiarism has been traditionally defined as the taking of words, images, processes, structure and design elements, ideas, etc. of others and presenting them as one’s own. It is often associated with phrases such as kidnapping of words, kidnapping of ideas, fraud, and literary theft. Plagiarism can manifest itself in a variety of ways and is not just confined to student papers or published articles or books. For example, consider a scientist who makes a presentation at a conference and discusses at length an idea or concept that had already been proposed by someone else yet not considered common knowledge. During his presentation, he fails to fully acknowledge the specific source of the idea and, consequently, misleads the audience into believing that he was the originator of that idea. This, too, may constitute an instance of plagiarism. The fact is that plagiarism manifests itself in a variety of situations and the following examples are just a small sample of the many ways in which it occurs and of the types of consequences that can follow as a result." (Source: U.S. Department of Health and Human Services (HHS) Office of Research Integrity (ORI): "Avoiding Plagiarism, Self-plagiarism, and Other Questionable Writing Practices: A Guide to Ethical Writing" "This guide was written by Miguel Roig, PhD, from St. Johns University with funding from ORI. This module was originally created in 2003 and revised in 2006 and 2015." View/Download pdf here) - "Plagiarism of ideas Appropriating someone else’s idea (e.g., an explanation, a theory, a conclusion, a hypothesis, a metaphor) in whole or in part, or with superficial modifications without giving credit to its originator." (Source: ibid.)
- Acknowledging the Source of Our Ideas
"The confidential peer review process is thought to be a common source of plagiarism. Consider the scenario where the offender is a journal or conference referee, or a member of a review panel for a funding agency. He reads a paper or a grant proposal describing a promising new methodology in an area of research directly related to his own work. The grant fails to get funded based perhaps on his negative evaluation of the protocol. He then goes back to his lab and prepares a grant proposal using the methodology stolen from the proposal that he refereed earlier and submits his proposal to a different granting agency. Cases similar to the above scenario have been documented in the research misconduct literature (see Price, 2006)
Most of us would deem the behavior depicted in the above scenario as downright despicable. Unfortunately, similar situations have occurred. In fact, elements of the above scenario are based on actual cases of scientific misconduct investigated by ORI. The notion that the peer review context appears to be sufficiently susceptible to the appropriation of ideas was likely the impetus behind the 1999 Federal Office of Science and Technology Policy’s expansion of their definition of plagiarism, which states:- “Plagiarism is the appropriation of another person’s ideas, processes, results, or words without giving appropriate credit, including those obtained through confidential review of others’ research proposals and manuscripts. (Office of Science and Technology Policy, 1999)." (Source: ibid.)
- 28 Guidelines at a Glance on Avoiding Plagiarism
The 28 guidelines (with URL links) listed below are taken from "Avoiding plagiarism, self-plagiarism, and other questionable writing practices: A guide to ethical writing" by Miguel Roig, as published on the website of U.S. Department of Health and Human Services (HHS) Office of Research Integrity (ORI).- Guideline 1: An ethical writer ALWAYS acknowledges the contributions of others to his/her work.
- Guideline 2: Any verbatim text taken from another source must be enclosed in quotation marks and be accompanied by a citation to indicate its origin.
- Guideline 3: When we summarize others’ work, we use our own words to condense and convey others’ contributions in a shorter version of the original.
- Guideline 4: When paraphrasing others’ work, not only must we use our own words, but we must also use our own syntactical structure.
- Guideline 5: Whether we are paraphrasing or summarizing we must always identify the source of our information.
- Guideline 6: When paraphrasing and/or summarizing others’ work we must ensure that we are reproducing the exact meaning of the other author’s ideas or facts and that we are doing so using our own words and sentence structure.
- Guideline 7: In order to be able to make the types of substantial modifications to the original text that result in a proper paraphrase, one must have a thorough command of the language and a good understanding of the ideas and terminology being used.
- Guideline 8: When in doubt as to whether a concept or fact is common knowledge, provide a citation.
- Guideline 9: Authors of complex studies should heed the advice previously put forth by Angell & Relman (1989). If the results of a single complex study are best presented as a ‘cohesive’ single whole, they should not be partitioned into individual papers. Furthermore, if there is any doubt as to whether a paper submitted for publication represents fragmented data, authors should enclose other papers (published or unpublished) that might be part of the paper under consideration (Kassirer & Angell, 1995).
- Guideline 10: Authors who submit a manuscript for publication containing previously disseminated data, reviews, conclusions, etc., must clearly indicate to the editors and readers the nature of the previous dissemination. The provenance of data must never be in doubt.
- Guideline 11: While there are some situations where text recycling is an acceptable practice, it may not be so in other situations. Authors are urged to adhere to the spirit of ethical writing and avoid reusing their own previously published text, unless it is done in a manner that alerts readers about the reuse or one that is consistent with standard scholarly conventions (e.g., by using of quotations and proper paraphrasing).
- Guideline 12: In the domain of conferences and similar audio-visual presentations of their work, authors should practice the same principles of transparency with their audiences.
- Guideline 13: In addition to standard practices of ethical scholarship, authors must be mindful of readers’ expectations, applicable issues related to intellectual content rights (i.e., copyright), and, especially, the need to always be transparent in our work when reusing material across the various dissemination domains.
- Guideline 14: Because some instances of plagiarism, self- plagiarism, and even some writing practices that might otherwise be acceptable (e.g., extensive paraphrasing or quoting of key elements of a book) can constitute copyright infringement, authors are strongly encouraged to become familiar with basic elements of copyright law.
- Guideline 15: Authors are strongly urged to double-check their citations. Specifically, authors should always ensure that each reference notation appearing in the body of the manuscript corresponds to the correct citation listed in the reference section and vice versa and that each source listed in the reference section has been cited at some point in the manuscript. In addition, authors should also ensure that all elements of a citation (e.g., spelling of authors’ names, volume number of journal, pagination) are derived directly from the original paper, rather than from a citation that appears on a secondary source. Finally, when appropriate, authors should ensure that credit is given to those authors who first reported the phenomenon being studied.
- Guideline 16: The references used in a paper should only be those that are directly related to its contents. The intentional inclusion of references of questionable relevance for purposes such as manipulating a journal’s or a paper’s impact factor or a paper’s chances of acceptance, is an unacceptable practice.
- Guideline 17: Always cite the actual work that is consulted. When the published paper cannot be obtained, cite the specific version of the material being used whether it is conference presentation, abstract, or an unpublished manuscript. Ensure that the cited work has not been subsequently corrected or retracted.
- Guideline 18: Generally, when describing others’ work, do not cite an original paper if you are only relying on a secondary summary of that paper. Doing so is a deceptive practice, reflects poor scholarly standards, and can lead to a flawed description of the work described.
- Guideline 19: If an author must rely on a secondary source (e.g., textbook) to describe the contents of a primary source (e.g., an empirical journal article), s/he should consult writing manuals used in her discipline to follow the proper convention to do so. Above all, always indicate to the reader the actual source of the information being reported.
- Guideline 20: When borrowing heavily from a source, authors should always craft their writing in a way that makes clear to readers which ideas/data are their own and which are derived from sources being consulted.
- Guideline 21: When appropriate, authors have an ethical responsibility to report evidence that runs contrary to their point of view. In addition, evidence that we use in support of our position must be methodologically sound. When citing supporting studies that suffer from methodological, statistical, or other types of shortcomings, such flaws must be pointed out to the reader.
- Guideline 22: Authors have an ethical obligation to report all aspects of the study that may impact the replicability of their research by independent observers.
- Guideline 23: Researchers have an ethical responsibility to report the results of their studies according to their a priori plans. Any post hoc manipulations that may alter the results initially obtained, such as the elimination of outliers or the use of alternative statistical techniques, must be clearly described along with an acceptable rationale for using such techniques.
- Guideline 24: Authorship determination should be discussed prior to commencing research collaboration and should be based on established guidelines, such as those of the International Committee of Medical Journal Editors.
- Guideline 25: Only those individuals who have made substantive contributions to a project merit authorship in a paper.
- Guideline 26: Faculty-student collaborations should follow the same criteria to establish authorship. Mentors must exercise great care to neither award authorship to students whose contributions do not merit it, nor to deny authorship and due credit to the work of students.
- Guideline 27: Academic or professional ghost authorship in the sciences is ethically unacceptable
- Guideline 28: Authors must become aware of possible conflicts of interest in their own research and to make every effort to disclose those situations (e.g., stock ownership, consulting agreements to the sponsoring organization) that may pose actual or potential conflicts of interest."
(Source: U.S. Department of Health and Human Services (HHS) Office of Research Integrity (ORI): "Avoiding Plagiarism, Self-plagiarism, and Other Questionable Writing Practices: A Guide to Ethical Writing" "This guide was written by Miguel Roig, PhD, from St. Johns University with funding from ORI. This module was originally created in 2003 and revised in 2006 and 2015." View/Download pdf here.)
Plagiarism (continued)
Plagiarism in Combination With Falsification and/or Fabrication
"Cases of Plagiarism Handled by the United States Office of Research Integrity 1992–2005"
- "Abstract
Since 1992, the Federal Office of Research Integrity has been making public findings of plagiarism as scientific misconduct against individuals involved in United States Public Health Service supported research. This paper is a historical review of the 19 ORI plagiarism cases, describing the characteristics of those respondents, the PHS administrative actions taken against them, the source of the plagiarized material, and the type of person who detected the plagiarism. Almost all of the 10 plagiarists debarred by ORI/PHS from Federal funding also falsified and/or fabricated research material, thereby compounding the seriousness of their plagiarism."
Following is the 1st of 11 Case Descriptions for Plagiarism in Combination With Falsification and/or Fabrication
"Freisheim – He was a professor of biochemistry and department chairman at the Medical College of Ohio, who plagiarized some research method designs on membrane‐transport of the anti‐cancer drug methotrexate, from the application of an assistant professor at another institution, for which he had been a confidential reviewer, into his own NIH grant application. When the original person, as an NIH reviewer, saw the questioned application, he raised concerns with the NIH review staff. On being informed of the allegation (without sequestration of evidence), the respondent fabricated a hand‐written document that he claimed to have circulated with his ideas to the research community a year before the source application was submitted. However, the complainant then provided an application with similar words and ideas that he had submitted to NIH a year earlier than the respondent’s purported document. Furthermore, the investigation committee found falsifications in the fabricated document, making clear that it could not have been written when the respondent had claimed. The institution forced him to resign. In 1993 ORI debarred him for 3 years, as well as required certification of future applications and prohibited his PHS service for 10 years. [14]
(...)
Conclusion
These data show that ORI has taken significant actions against 19 plagiarists (interestingly, all of whom were male scientists [26]) in PHS-related research, who have misappropriated words or ideas from the grant applications or publications of other scientists without crediting the source. ORI took the most severe actions against plagiarists who also falsified or fabricated data or documents, generally debarring them from Federal funding for 3 to 5 years. [27]
However, no matter how severe the administrative actions imposed, ORI publishes the name of the respondent, institution, and description of the detailed findings of scientific misconduct in:- Federal Register: http://www.gpoaccess.gov/fr/
- NIH Guide to Grants and Contracts: http://grants.nih.gov/grants/guide/index.html
- ORI Case Summaries: http://ori.hhs.gov/misconduct/cases/index.shtml
- ORI Newsletter: http://ori.hhs.gov/publications/newsletters.shtml
- ORI Annual Report: http://ori.hhs.gov/publications/annual_reports.shtml
- for those under current PHS actions, PHS Administrative Actions Bulletin Board: http://ori.hhs.gov/publications/annual_reports.shtml
- for those under current PHS actions, PHS Administrative Actions Bulletin Board: http://silk.nih.gov/public/[email protected]
- and for those debarred, Federal Excluded Persons List System: http://epls.arnet.gov/
- all of which can be searched on the Internet. [28] Indeed, a Google search on "First-Name Last-Name" and NIH Guide (or ORI) generally pulls up the NIH Guide and/or ORI Website listing for the respondent at the top of the list. Thus, it is possible for other scientists and institutional officials to check easily for any ORI findings and currently active administrative actions against a specific person before making a decision on hiring or working with that person." (Source: "Cases of Plagiarism Handled by the United States Office of Research Integrity 1992–2005" Alan R. Price, Associate Director for Investigative Oversight, Office of Research Integrity. Plagiary: Cross‐Disciplinary Studies in Plagiarism, Fabrication, and Falsification, 46‐56. [Access HTML version here.])
Plagiarism (continued)
Definitions
- "In academic writing, it is considered plagiarism to draw any idea or any language from someone else without adequately crediting that source in your paper. It doesn't matter whether the source is a published author, another student, a Web site without clear authorship, a Web site that sells academic papers, or any other person: Taking credit for anyone else's work is stealing, and it is unacceptable in all academic situations, whether you do it intentionally or by accident." (Source: "What Constitutes Plagiarism?" in Harvard Guide to Using Sources)
- "Plagiarism is presenting someone else’s work or ideas as your own, with or without their consent, by incorporating it into your work without full acknowledgement. All published and unpublished material, whether in manuscript, printed or electronic form, is covered under this definition. Plagiarism may be intentional or reckless, or unintentional. Under the regulations for examinations, intentional or reckless plagiarism is a disciplinary offence." (Source: University of Oxford Website: https://www.ox.ac.uk/students/academic/guidance/skills/plagiarism)
- "The action or practice of taking someone else's work, idea, etc., and passing it off as one's own; literary theft." The verb “to plagiarize” is defined as: “To take and use as one's own (the thoughts, writings, or inventions of another person);” “To copy (literary work or ideas) improperly or without acknowledgement; (occas.) to pass off as one's own the thoughts or work of (another)” (Source: Oxford English Dictionary, 2011)
- "taking over the ideas, methods, or written words of another, without acknowledgment and with the intention that they be taken as the work of the deceiver." (Source: American Association of University Professors (September/October, 1989)).
- "the act of using another person's words or ideas without giving credit to that person : the act of plagiarizing something.” (Source: Merriam-Webster: plagiarism)
- "plagiarism– the use of published or unpublished material without due acknowledgment of the primary author– constitutes research misconduct in the same way as does the fabrication and/or the falsification of data." (Source:"Fraud and deceit in medical research," Umran Sarwar and Marios Nicolaou, J Res Med Sci. 2012 Nov; 17(11): 1077–1081. PMCID: PMC3702092)
Plagiarism (continued)
- Council of Science Editors (CSE)
"White Paper on Publication Ethics"
"3.1.3 Piracy and Plagiarism
Piracy is defined as the unauthorized reproduction or use of ideas, data, or methods from others without adequate permission or acknowledgment. Again, deceit plays a central role in this form of misconduct. The intent of the perpetrator is the untruthful portrayal of the ideas or methods as his or her own. 2
Plagiarism is a form of piracy that involves the unauthorized use or close imitation of the language (figures images or tables) and thoughts of others and the representation of them as one’s own original work without permission or acknowledgment by the author of the source of these materials. Plagiarism generally involves the use of materials from others, but can apply to researchers’ duplication of their own previously published reports without acknowledgment (this is sometimes called self-plagiarism or duplicate publication).2 Sample correspondence is available on the CSE website. 5" (Source: "White Paper on Publication Ethics" Copyright © 2018 Council of Science Editors. (Download a PDF of the entire White Paper))
Plagiarism (continued)
"Plagiarism: Why is it such a big issue for medical writers?"
- "Plagiarism of ideas
Even if an author does not copy any words and phrases from the original article, if he simply uses the same idea, thought, or invention and presents it as his own without proper acknowledgment, the same may amount to plagiarism. This kind of plagiarism is difficult to detect[10] but once detected, it is as serious an offence.
When can plagiarism of an idea occur? Here are a few examples that come to mind. It is possible that after a particular article has been rejected by a board of reviewers, one of the reviewers may “kidnap” the idea, write a fresh article, and get it published in a different journal under his name. This is plagiarism of idea.
(...)
Plagiarism of ideas is also common during seminar and conference presentations. The presenters often pick up ideas from various sources such as text books, research journals, conference proceedings, etc., and compile a presentation on a particular topic and present it as their own. As long as all sources are aptly acknowledged in the presentation, it is fair play. However, any idea or thought that is not adequately attributed to its rightful owner, whether intentionally or unintentionally amounts to plagiarism of ideas." (Source: "Plagiarism: Why is it such a big issue for medical writers?" Natasha Das and Monica Panjabi. Perspect Clin Res. 2011 Apr-Jun; 2(2): 67–71. doi: [10.4103/2229-3485.80370])
"Responding to Possible Plagiarism"
"Documenting reactions from authors and journal editors to plagiarism may help others address the problem."
- "The peer-review process is the best mechanism to ensure the high quality of scientific publications. However, recent studies have demonstrated that the lack of well-defined publication standards, compounded by publication process failures (1), has resulted in the inadvertent publication of several duplicated and plagiarized articles."
(...)
While there will always be a need for authoritative oversight, the responsibility for research integrity ultimately lies in the hands of the scientific community. Educators and advisors must ensure that the students they mentor understand the importance of scientific integrity. Authors must all commit to both the novelty and accuracy of the work they report. Volunteers who agree to provide peer review must accept the responsibility of an informed, thorough, and conscientious review. Finally, journal editors, many of whom are distinguished scientists themselves, must not merely trust in, but also verify the originality of the manuscripts they publish." (Source: "Responding to Possible Plagiarism" Tara C. Long, Mounir Errami, Angela C. George, Zhaohui Sun, Harold R. Garner. Science 06 Mar 2009: Vol. 323, Issue 5919, pp. 1293-1294. DOI: 10.1126/science.1167408)
"Authorship: The Coin of the Realm, The Source of Complaints"
- "CREDIT for one's research, ultimately through authorship, is extremely important for a successful scientific career. It affects future research funding, promotions, and recruitment opportunities. If allocation of credit is poorly done, future research resources may in turn be misallocated." (Source: "Authorship: The Coin of the Realm, The Source of Complaints" Linda J. Wilcox, EdM, CAS. JAMA. 1998;280(3):216-217. doi:10.1001/jama.280.3.216)
"Even potential participants of a research integrity conference commit plagiarism, organizers learn"
- "So the final diagnosis is that we had a bit more than 2% suspected plagiarism and somewhat less than 5% suspected self-plagiarism. It’s clear that plagiarism is not permissible. The importance of avoiding self-plagiarism is less obvious. The recently revised Netherlands Code of Conduct on Research Integrity labels it as a questionable research practice if it’s more than reuse on a small scale or of introductory passages and descriptions of the method applied. A conference abstract is not necessarily considered ‘published material’ but may be treated as ‘working paper’ with continuous improvement until the work is finally published. We do not know how often largely identical abstracts are submitted by their authors to multiple conferences, but we believe that if that happens it should be disclosed to reviewers and conference participants.
Somewhat sadder and wiser we have to conclude that potential participants of a research integrity conference are not immune for at least one form of research misbehaviour." (Source: "Even potential participants of a research integrity conference commit plagiarism, organizers learn" Retraction Watch, Lex Bouter. January 10, 2019)
"One would hope that researchers submitting abstracts for a meeting on research integrity would be less likely to commit research misconduct. But if the experience of the 6th World Conference on Research Integrity is any indication, that may not be the case. Here, the co-organizers of the conference — Lex Bouter, Daniel Barr, and Mai Har Sham — explain."
NORWEGIAN MINISTRY OF EDUCATION AND RESEARCH
Consultation on revision of the Research Ethics Act (Høring om revisjon av forskningsetikkloven)
"Consultation paper – Research Ethics in Norway" (p. 36)
- "Plagiarism in a research ethics context means using others' texts, figures, tables, results, ideas, methods, processes and similar without indicating this and without listing the source. The most common definition of plagiarism is publishing others' work/texts as one's own, and thereby mislead the reader as to who wrote the text. In a research ethics context, it has been common to refer to Item 28 of the NESH Guidelines, which defines plagiarism as follows:
- "Plagiarism is, in a research ethics context, stealing content from other authors' and researchers' work and publishing it as one's own. Researchers who use others' ideas or quote from publications or research materials, must list their sources. The most severe form of plagiarism is pure copying. However, plagiarism can also come in other, more refined forms, and can apply for limited results, ideas, hypotheses, terms, theories, interpretations, design, etc. Referring to another work at an early point in one's own text, and then making extensive use of it without further references, is also plagiarism."
Norway's Research Ethics Act
- Section 8. Statements in cases of fraud
"Scientific dishonesty means falsification, fabrication, plagiarism and other serious violations of recognized research ethical norms committed intentionally or through gross negligence in planning, conducting or reporting research." (Source: Forskningsetikkloven (Research Ethics Act); English Translation: Research Ethics Act - "The Act states that there shall be research ethics committees covering all disciplines (§ 9). The national committees are The National Committee for Medical and Health Research Ethics (NEM); The National Committee for Research Ethics in Science and Technology (NENT); and The National Committee for Research Ethics in the Social Sciences and the Humanities (NESH); as well as the The National Commission for the Investigation of Research Misconduct (GRU)." (Source: "The Act on ethics and integrity in research" Text: The Norwegian National Committees for Research Ethics. Last updated: Monday, February 12, 2018)
- "Scientific Integrity
"The researcher is responsible for respecting the research results of others and to conduct good scientific practice. The researcher shall not conceal, distort or falsify, whether in the planning, implementation or reporting of the research. Plagiarism involves representing other people's ideas or research as one's own."
"Vitenskapelig redelighet
Forskeren har ansvar for å respektere andres forskningsresultater og å utøve god vitenskapelig praksis. Forskeren skal ikke skjule, fordreie eller forfalske noe, enten det er i planleggingen, gjennomføringen eller rapporteringen av forskningen. Å plagiere innebærer å framstille andres ideer eller forskning som sitt eget."
(Source: "Research ethical guidelines for natural sciences and technology" ("Forskningsetiske retningslinjer for naturvitenskap og teknologi") NENT • Den nasjonale forskningsetiske komité for naturvitenskap og teknologi. Oslo, april 2016 Øyvind Mikkelsen, Komitéleder. 2. utgave – april 2016. ISBN: 978-82-7682-073-7) - "The extent of fraudulent research (...)
In Finland and Norway, cases of plagiarism are clearly dominant in the complaints about possible dishonesty and even more dominant in terms of the conclusion of scientific dishonesty."
"Omfanget av uredelig forskning (...)
I Finland og Norge er saker om plagiering klart dominerende blant klagene om mulig uredelighet og enda mer dominerende når det gjelder konklusjon om vitenskapelig uredelighet."
(Source: "Fraud and plagiarism" "Fusk og plagiering" Research Ethics Library - FBIB (Forskningsetisk bibliotek - FBIB) Text: Torkild Vinther. Last updated: January 11, 2016) - Plagiarism (...)
"Among the Norwegian irregularities that the Examination Committee has knowledge of, either from its own treatment or through information from institutions, a significant number have been about plagiarism. Not least, it is important to note that virtually all issues the last approx. 8 years where it has been concluded with a form of scientific dishonesty, it has been primarily about plagiarism. There is therefore a good reason to focus on this..."
"Plagiering (...)
Blant de norske uredelighetssaker som Granskingsutvalget har kjennskap til, enten fra egen behandling eller via orientering fra institusjoner, har riktig mange vedrørt plagiering. Ikke minst er det viktig å bemerke at stort sett alle saker de siste ca. 8 år hvor det er konkludert med en form for vitenskapelig uredelighet primært har dreiet seg om plagiering. Det er altså god grunn til å sette fokus på dette..." (Source: ibid.)
- "In Finland and Norway, plagiarism cases clearly dominate in the reports of possible misconduct, and even more so with regard to conclusions of research misconduct. In Denmark, cases pertaining to FF [Falsification & Febrication] account for nearly half of all FFP [Falsification, Fabrication & Plagiarism] cases.
(...)
The number of allegations or cases that are put forward or dealt with is an indication of the extent of the problem. A series of studies, mainly from abroad but including a single Norwegian study, show that the extent of misconduct and questionable conduct is relatively large. (Source: "Fraud and plagiarism" Torkild Vinther. Last updated: 25. February 2016. The Norwegian National Research Ethics Committees)
"Dear peer reviewer, you stole my paper: An author’s worst nightmare"
- "Deeply disturbing," "heinous intellectual theft," erosion of the "public’s trust in medical research:" These are just a few -words used to describe a rare type of plagiarism reported in this week’s Annals of Internal Medicine."
"Although we’ve only documented a few cases where peer reviewers steal material from manuscripts and pass them off as their own, it does happen, and it’s a fear of many authors. What we’ve never seen is a plagiarized author publish a letter to the reviewer who stole his work. But after Michael Dansinger of Tufts Medical Center realized a paper he’d submitted to Annals of Internal Medicine that had been rejected was republished, and the journal recognized one of the reviewers among the list of co-authors, it published a letter from Dansinger to the reviewer, along with an editorial explaining what happened.
The letter and editorial identify the paper containing the stolen material — now retracted — but don’t name the reviewer responsible. Still, the articles are deeply personal. As Dansinger writes in “Dear Plagiarist: A Letter to a Peer Reviewer Who Stole and Published Our Manuscript as His Own,” the reviewer took much more than just a manuscript:
It took 5 years from conceptualization of the study to publication of the primary analysis (1). This study was my fellowship project and required a lot of work. It took effort to find the right research team, design the study, raise the funds, get approvals, recruit and create materials for study participants, run the diet classes, conduct the study visits, compile and analyze the study data, and write the initial report. The work was funded by the U.S. government and my academic institution. The secondary analysis that you reviewed for Annals used specialized methods that took my colleagues many years to develop and validate. In all, this body of research represents at least 4000 hours of work."
"In “Scientific Misconduct Hurts,” Annals of Internal Medicine. Editor-in-Chief Christine Laine identifies the “several layers of bold misconduct” that took place..." (Source: "Dear peer reviewer, you stole my paper: An author’s worst nightmare" Alison McCook. Retraction Watch December 12, 2016)
Also see: "Caught Our Notice: 4th retraction for peer reviewer who stole manuscript" (Source: Retraction Watch)
"Chem journal yanks paper because authors had stolen it as peer reviewers"
- "The UK’s Royal Society of Chemistry has retracted a 2017 paper in one of its journals after learning that the authors stole the article from other researchers during peer review." (Source: "Chem journal yanks paper because authors had stolen it as peer reviewers" Adam Marcus. Posted on February 8, 2019 Retraction Watch)
- "This is not, we should note, the first time we have reported on a case like this. Here are several other examples:
- “I am really sorry:” Peer reviewer stole text for own paper
- Dear peer reviewer, you stole my paper: An author’s worst nightmare, followed by three more retractions for the same author
- Yikes: Peer reviewer stole (and published) author’s data
- Peer reviewer steals text for his own chemistry paper, gets sanctioned by journal
- Nightmare scenario: Text stolen from manuscript during review"
(Source: "Chem journal yanks paper because authors had stolen it as peer reviewers" Adam Marcus. Retraction Watch February 8, 2019. Retraction Watch)
"Peer reviewer stole article and published it as his own"
- "A peer reviewer appointed by the Annals of Internal Medicine stole research submitted by a team from Tufts University, Boston, that was rejected by the Annals and published it six months later in a different journal under his own name.
The theft came to light when the original lead author, the endocrinologist Michael Dansinger of Tufts, later chanced upon his plagiarized work in EXCLI Journal while searching the internet and contacted the corresponding author, who apologized for the plagiarism and had the article retracted. 1
(...)
Dansinger contacted the corresponding author, Carmine Finelli, after finding the plagiarism online. Finelli admitted the plagiarism, did not offer an explanation, and said that he would retract the EXCLI Journal article, which he did.
The retraction notice read, “As corresponding author I ask for retraction of our article Finelli et al (2016) with the consent of all co-authors, because of unauthorized reproduction of confidential content of another manuscript." (Source: "Peer reviewer stole article and published it as his own" Owen Dyer. BMJ 2016; 355:i6768 doi: https://doi.org/10.1136/bmj.i6768 (Published 19 December 2016)
"knowingly, intentionally, or recklessly [appropriated] the ideas, processes, results or words”
"Prominent US critic of cancer screening committed plagiarism, investigation finds"
- "A well known scholar in US healthcare policy plagiarised his colleague at Dartmouth College, New Hampshire, in a widely cited critique of breast cancer screening, an internal investigation by the college has found.
H Gilbert Welch “engaged in research misconduct, namely, plagiarism,” the investigation committee found, in writing a paper published in the New England Journal of Medicine (NEJM). 1 The article, which questioned the survival benefits of breast cancer screening, attracted widespread media attention and was cited by 94 other articles, making it one of the top 1% of influential research articles of 2016.
The committee found that Welch, a professor at the Geisel School of Medicine and the Dartmouth Institute for Health Policy and Clinical Practice, had “knowingly, intentionally, or recklessly [appropriated] the ideas, processes, results or words” of Dartmouth associate professor Samir Soneji and his colleague Hiram Beltrán-Sánchez, an associate professor at the University of California, Los Angeles." (Source: "Prominent US critic of cancer screening committed plagiarism, investigation finds" Owen Dyer. BMJ 2018;362:k3616 doi: 10.1136/bmj.k3616 Published 22 August 2018) [Also see: Prominent health policy researcher resigns from Dartmouth over plagiarism dispute]
Plagiarism (continued)
"Background: Plagiarism is more than just copying someone else's text"
"Bakgrunn: Plagiat er mer enn bare å kopiere en annens tekst"
- "You do not have to write the text to commit ethical theft. - The use of other people's ideas, hypotheses, interpretations, illustrations or results is equally wrong, according to researchers."
"Du trenger ikke å skrive av teksten for begå forskningsetisk tyveri. – Bruk av andres idéer, hypoteser, tolkninger, illustrasjoner eller resultater er like galt, ifølge forsker."
Basic ethical norms
Grunnleggende etiske normer
"A professional book or a scientific article is the spiritual product of the researcher, which he owns and to which he has copyright.
"En fagbok eller en vitenskapelig artikkel er forskerens åndelige produkt, som vedkommende eier og har opphavsrett til.
The ideas and results that the book or article explains may be used by others, but the rules of the game are clear: everyone who borrows these ideas and results must state where it comes from. That is, give a clear and clear source reference. Otherwise, the document is considered unlawful use after the research ethics.
Ideene og resultatene som boken eller artikkelen redegjør for, kan godt brukes av andre, men spillereglene er klare: alle som låner disse ideene og resultatene, må oppgi hvor det kommer fra. Det vil si gi en klar og tydelig kildehenvisning. Ellers betraktes handlingen som urettmessig bruk etter forskningsetikken.
And just like in literature or music, unfair use of others' achievements in research constitutes plagiarism.
Og akkurat som i litteraturen eller musikken, omtales urettmessig bruk av andres prestasjoner i forskningssammenheng som plagiat.
In Norway, research ethics committees have been established which have developed ethical guidelines for various disciplines. According to their definition, plagiarism in the research ethical sense is "taking something from others and presenting it as their own without a good reference to the sources".
I Norge er det etablert forskningsetiske komitéer som har utarbeidet etiske retningslinjer for ulike fagområder. Ifølge deres definisjon er plagiat i forskningsetisk forstand «å ta noe fra andre og presentere det som sitt eget uten god henvisning til kildene».
"The most obvious plagiarism in the world of research is copying," says Irgens-Jensen.
Det mest åpenbare plagiat i forskningens verden er avskrift, forteller Irgens-Jensen.
"It is obviously easy to discover and is completely reprehensible. It will also be in violation of the intellectual property laws. Nevertheless, a number of examples have been identified where texts were written without reference recently. A case that wakened international attention was a major plagiarism in the legal doctorate of the then German defense minister Karl-Theodor zu Guttenberg. He was deprived of his PhD in 2011 and had to resign from his position."
– Det er naturligvis lett å oppdage og er helt forkastelig. Det vil også være i strid med åndsverkloven. Likevel er det blitt identifisert en rekke eksempler hvor det ble skrevet av tekster uten henvisning i den senere tid. En sak som vekket internasjonal oppsikt, var en omfattende plagiering i den juridiske doktorgraden til den daværende tyske forsvarsministeren Karl-Theodor zu Guttenberg. Han ble fratatt sin doktorgrad i 2011 og måtte fratre sin stilling."
What falls under the radar of the law
Det som faller under jussens radar.
"Plagiarism, however, is not always obvious. In addition to concrete and robust offenses - such as the obvious transcript, as mentioned above - there are other types of "thefts" that are considerably more difficult to detect.
Plagiat er imidlertid ikke alltid innlysende. Utover konkret og håndfast lovbrudd – som for eksempel den åpenbare avskriften, som nevnt over – fins det andre typer «tyverier», som er atskillig vanskeligere å oppdage.
- For example, where the offender uses his own formulations, yet has retrieved the ideas and research results from others without giving up the source. You do not have to write the text to commit ethical theft. The use of other people's ideas, hypotheses, interpretations, illustrations or results is equally wrong, even though it is more difficult to detect.
– Eksempelvis der krenkeren bruker sine egne formuleringer, men likevel har hentet ideene og forskningsresultatene fra andre uten å oppgi kilden. Man trenger ikke å skrive av teksten for begå forskningsetisk tyveri. Bruk av andres idéer, hypoteser, tolkninger, illustrasjoner eller resultater er like galt, selv om det er vanskeligere å oppdage.
"It is important to counteract various degrees of dishonesty, from sloppiness to cheating. The honest student, researcher or author has good opportunities to do it in a neat manner. He or she can credit the author and that the text or ideas are taken from an existing work," concludes Irgens-Jensen.
– Det er viktig å motarbeide ulike grader av uredelighet, fra sjusk til fusk. Den redelige studenten, forskeren eller forfatteren har gode muligheter til å gjøre det på en ryddig måte. Han eller hun kan kreditere opphavsmannen, og oppi at teksten eller ideene er hentet fra et eksisterende verk, avslutter Irgens-Jensen. (Source: "Background: Plagiarism is more than just copying someone else's text" "Bakgrunn: Plagiat er mer enn bare å kopiere en annens tekst"Associate Professor Harald Irgens-Jensen, University of Oslo. forskning.no. PUBLISHED December 02. 2017)
Plagiarism (continued)
Committee on Publication Ethics (COPE)
"What to do if you suspect plagiarism"
- (a) Suspected plagiarism in a submitted manuscript
- (b) Suspected plagiarism in a published article
- How should editors respond to plagiarism? (COPE discussion paper, © COPE, April 2011)
- "COPE: Systematic Manipulation of the Publication Process"
Plagiarism (continued)
"A colleague included plagiarized material in your grant proposal. Are you liable?"
- "Picture this scenario: You submit an NIH grant proposal. Unbeknownst to you, it contains material plagiarized from another scientist. Are you liable for research misconduct?
"The answer is clearly yes.” That’s according to a recent decision by Administrative Law Judge Keith Sickendick, of the U.S. Department of Health and Human Services, in a case involving a former University of Kansas Medical Center professor." (Source: "A colleague included plagiarized material in your grant proposal. Are you liable?" Richard Goldstein. Retraction Watch. December 3, 2018)
Plagiarism (continued)
Public exposure of plagiarists
"Iain Chalmers describes a case of scientific misconduct in which an author plagiarised both text and data on two separate instances.1 Although he took the proper steps after detecting the plagiarism, Chalmers seems to have been unsuccessful in resolving the matter. This and other similar failures of our scientific system of justice leads me to support his recommendations for dealing with plagiarism, particularly his call to publicly expose those who have been found guilty of misconduct.
Public exposure of plagiarists, and the consequent embarrassment and ostracism that these offenders should experience, not only satisfies our intrinsic need for social justice but can also serve as a deterrent. Unfortunately, many scientific journals, professional organisations, and academic institutions lack the necessary resources and, apparently in some cases, even the will to investigate misconduct allegations." (Source: "Ethical writing should be taught" Miguel Roig. BMJ 2006;333:596 doi: https://doi.org/10.1136/bmj.38946.501215.68 Published 14 September 2006. ) [Note: Citation 1: Chalmers I. Role of systematic reviews in detecting plagiarism: case of Asim Kurjak. BMJ 2006;333: 594–6. View/download PDF here. "Naming and shaming" and "Action" excerpts are included below.]
"Naming and shaming
Although this is but one case study [case of Asim Kurjak], it prompts me to make some general suggestions for detecting and reducing plagiarism. Firstly, journals and institutions should heed Nature’s editorial call 1 to take allegations of plagiarism more seriously than they seem to at present. Secondly, as has been suggested by Tom Jefferson and colleagues,9 10 journals should use systematic reviews for editorial peer review, as well as considering using software designed to detect plagiarism. Lastly, to reduce the numbers of new as well as recurrent plagiarists, journals, institutions, and professional associations need to expose very publicly those found guilty of this form of scientific misconduct." (Source : Analysis And Comment, Professional regulation, "Role of systematic reviews in detecting plagiarism: case of Asim Kurjak" Iain Chalmers. BMJ 2006;333:594. p. 595-596. (Published 14 September 2006) doi: https://doi.org/10.1136/bmj.38968.611296.F7. View/download PDF here.) [Note: Citation 1: "Complacency about misconduct" Editorial, Nature volume 427, page 1. 01 January 2004)
"Action
More than 15 years have passed since I detected Professor Kurjak’s plagiarism and reported it to the relevant authorities. Yet, as the Norwegian case shows, he seems to believe that he can continue to act with impunity in this respect. This continued scientific misconduct raises serious doubt about the extent to which his publications are trustworthy accounts of his own work. Despite this record of scientific misconduct, Professor Kurjak is clearly regarded with great respect, not only within Croatia but also by many obstetricians elsewhere. The case I have described is an illustration of a more widespread problem. What could or should a whistleblower like me expect from institutions and journals when plagiarism is reported to them. I can illustrate some general principles by itemising my current expectations in this case:
- The University of Zagreb should take steps to check or ensure that the 243 publications currently listed in Medline with A Kurjak as an author can be shown to be trustworthy accounts of Professor Kurjak’s work
- The University of Zagreb should make publicly available, in English, an account of the methods, findings, and conclusions of its investigation
- The university should send the results of its investigation to all the journals and editors of books containing publications by Professor Kurjak, to those who have purportedly coauthored articles with him, and to all the officers of the International Society for Ultrasound in Obstetrics and Gynecology
- Journals containing articles of dubious authenticity authored by Professor Kurjak should publish notices drawing attention to these concerns and notify the relevant bibliographic databases (the Medline record (PMID: 4820193) of the 1974 Kurjak and Beazley article published in Acta Medica Iugoslavica has no annotation, only a list of 10 related articles).
"Plagiarism and punishment"
"The Kurjak plagiarism case: the vicious circle of academic corruption
The cloud now hanging over Croatia's research community (1) is dark indeed. The Court of Honour of Zagreb University’s Medical School has dismissed proven allegations of Professor Kurjak's misconduct (2) on the
grounds that his misconduct had occurred long ago, that the culprit had already apologised, and that he recently retired (3). The decision of the Court of Honour did not refer to the fact that the national Committee for Ethics in Science and Higher Education of the Republic of Croatia (4) had confirmed the allegations made in Chalmers’ article published in the BMJ last year (2), and had found additional examples of Kurjak’s unethical practices after evaluating his whole publication opus (5). Nor did the Medical School’s Court of Honour mention that, in July this year, the Ministry of Science, Education and Sports suspended the funding of Kurjak's research grant (6).
(...)
The Economist recently suggested that “the concept of conflict of interest is little understood” in Croatia (12). The actions of the University of Zagreb and the Medical School suggest that the academic community in Zagreb believes that its autonomy provides it with immunity from its responsibility to the public. Invited to comment on Kurjak’s misconduct during a TV show, University Rector Bjelis stated that plagiarism was a benign problem and that the University had more important problems to address. Institutional failure to deal with research misconduct occurs in other academic communities (13), but a worrying aspect of the failures described above is their possible impact on the prospects for Croatia’s accession to the European Union (14). (Source: "Plagiarism and punishment" "The Kurjak plagiarism case: the vicious circle of academic corruption" Matko Marusic, Editor in Chief, Croatian Medical Journal, Zagreb, Croatia. BMJ 2007; 335:0 doi: https://doi.org/10.1136/bmj.39392.602523.47 (Published 08 November 2007))
"Croatia is let down"
"In November 2006 the editors of the Croatian Medical Journal asked the Committee on Publication Ethics (COPE) to help it in dealing with allegations against Asim Kurjak of duplicate or redundant publication, or both. These were quite separate from the previous allegations of plagiarism by this author.
An investigation by COPE concluded in February 2007, in regard to the papers submitted to it by the journal, that there is strong evidence that Kurjak (or his co-authors) committed publication misconduct on at least three occasions in relation to papers submitted to the Croatian Medical Journal. In two cases, papers co-authored by Kurjak were submitted to the journal after similar papers had been accepted by another journal, and in one case the material published in the Croatian Medical Journal seems to have been inappropriately republished in another journal.
COPE advised the editors of the Croatian Medical Journal to send its report to the University of Zagreb, requesting that it conduct an inquiry into the allegations.
One problem that has beset COPE since it was founded in 1997 has been the apparent reluctance of some institutions to take seriously complaints made to them by editors about probable publication misconduct by their staff or employees. The University of Zagreb now seems to be one of them. COPE is appalled by Marusic’s revelation (previous letter)1 that the report was swept under the carpet by those entrusted with maintaining the integrity of research at the university’s medical school.
Far from protecting the name of the university and its medical school, this action only serves to diminish their reputations and to cast doubt on the undoubted body of reliable and honest research carried out there.
COPE has written to the dean of the medical school, the rector of the university, and the Croatian minister of health, Professor Primorac, expressing its dismay. Let us hope that the minister directs that the court of honour at Zagreb University be reconvened to consider the complaint from the Croatian Medical Journal." (Source: "Croatia is let down" "The Kurjak plagiarism case" Harvey Marcovitch, Past Chairman of COPE. BMJ. 2008 Jan 26; 336(7637): 174. doi: 10.1136/bmj.39450.661528.3A.)
Plagiarism (continued)
- Intelligent Plagiarism
"What is worse, in my opinion, but was not discussed in these Nature articles, are cases where scientists rewrite previous findings in different words, purposely hiding the sources of their ideas, and then during subsequent years forcefully claim that they have discovered new phenomena. Such ‘intelligent plagiarism’ is, unfortunately, often more successful because most scientists do not have either time or sufficient interest to carefully investigate where the original results came from.
As such misconduct seems to me to have recently increased within the scientific community, I think that a thorough discussion of these issues, in Nature or elsewhere, is urgently needed." (Source: "Intelligent plagiarists are the most dangerous" Lennart Stenflo, Department of Physics, Umea University, SE-90187 Umea, Sweden. Nature 427, 777. 26 February 2004. DOI: https://doi.org/10.1038/427777a. pdf here)
Plagiarism (continued)
"Researchers accuse German authority of plagiarism in glyphosate review"
New report casts further doubt on EU’s glyphosate assessments.
- "The practice of copy paste and plagiarism ... influenced the authority's conclusions" — Report by Stefan Weber and Helmut Burtscher-Schaden (Source: "Researchers accuse German authority of plagiarism in glyphosate review" By SIMON MARKS 1/15/19, 5:34 PM CET Updated 1/24/19, 6:32 PM CET. POLITICO Europe Edition) [Note: Also see: "Why Plagiarism Matters" By Jonathan Bailey. PlagiarismToday January 17, 2019.]
- "Conclusion
The study authors’ analyses, in particular their detailed analysis of the chapters on carcinogenicity, suggest that the BfR‘s practice of copy paste and plagiarism is at odds with an independent, objective, and transparent assessment of the risks, and that this practice influenced the authority’s conclusions on glyphosate’s safety. In addition, the study authors found clear evidence of BfR’s deliberate pre - tence of an independent assessment, whereas in reality the authority was only echoing the industry applicants’ assessment." (Source: "Detailed Expert Report on Plagiarism and superordinated Copy Paste in the Renewal Assessment Report (RAR) on Glyphosat" Stefan Weber (Salzburg/Dresden) and Helmut Burtscher-Schaden (Vienna) 2019; In collaboration with Till Radinger (Cologne, Plagiarism Documentation Support) and Hannes Fuß (Berlin, Visualisation); Peer reviewed by Jonathan Bailey (New Orleans, PlagiarismToday) and Gerhard Dannemann (Berlin, Humboldt University; VroniPlag Wiki) © January 2019. p. 8) [Note: Also see: 15.01.2019 Letter from BfR to Doz. Dr. Stefan Weber.]
"4) What conclusions can be drawn from this copy paste and plagiarism analysis with regard to the statement by the head of the pesticides unit at the EFSA that there is no copy paste in Volume 1 of the RAR?
This statement is wrong. There seem to be two possible reasons for it: Stating a lie or a lack of knowledge (wrong briefing from the team).
5) In our opinion, what might be the reasons for the BfR’s approach, based on our experience and expertise in the field of plagiarism? And is there evidence of deliberate deception of the reader?
It is not possible to look into someone’s mind and therefore we do not know what motivated the responsible BfR staff to take this problematic approach. In principle, however, plagiarism can usually be traced back to one of the following two motives, or a combination of both:
1) Plagiarism makes it possible to achieve a desired result, which could otherwise only be achieved with significantly greater use of time and resources.
2) Plagiarism makes it possible to achieve a result that would otherwise not have been achievable at all, due to a lack of the necessary skills.
Given the huge amount of industry studies (in the Monsanto Hearing, Jose Tarazona spoke of “several hundred thousand” pages), the rapid progress of science, and the broad thematic range of published studies of possible relevance for the assessment, both the above explanations seem plausible.
In our opinion, the question of whether the BfR intended to deceive the reader must be answered with a clear “yes”. Clear indications of deception were found. Most striking was the finding that what the BfR described as the “approach taken by the RMS” was actually copy pasted from the GTF application and was the approach taken by Monsanto scientists. - "6) What conclusions can be drawn from this copy paste and plagiarism analysis with regard to the legally required 44 independence, objectivity, and transparency of the glyphosate evaluation?
With regard to the assessment performed by the BfR, the institute’s word-for-word adoption of the manufacturers’ assessments (“Klimisch evaluation”) of published studies in every single case can be only regarded as the opposite of independence. Because independence is a prerequisite for objectivity, the BfR’s assessment also lacks objectivity. Last but not least, the systematic omission of references to the real author via selective deletions can only be interpreted as deliberate concealment of the origin of the text. It goes without saying that this is the opposite of what we would expect from a transparent assessment.
However, with regard to the assessment performed by the UBA, the present analysis provided no evidence to cast doubt on the independence of the evaluation." (Source: Ibid., p. 53-54)
"Journal to retract article from 2000 that plagiarized one from 1984"
- "When it comes to plagiarism, there is apparently no statute of limitations."
"That’s one lesson one might take from this tale of two papers, one published in 1984 in the American Journal of Obstetrics and Gynecology (AJOG), and the other published in 2000 in the Medical Journal of The Islamic Republic of Iran (MJIRI). Both are titled “The use of breast stimulation to prevent postdate pregnancy.”" (Source: "Journal to retract article from 2000 that plagiarized one from 1984" Ivan Oransky. Retraction Watch February 22, 2019)
"Analysis of retractions in Indian science"
- "Abstract
An increasing problem throughout the world, plagiarism and related dishonest behaviors have been affecting Indian science for quite some time. To curb this problem, the Indian government has initiated a number of measures, such as providing plagiarism detecting software to all the universities for free. Still, however, many unfair or incorrect papers are published. For some time, publishers have used an efficient tool to deal with such situations: retractions.
(...)
Thus, we analyzed 239 retractions (195 from journals and 44 from conference proceedings) published between 2005 and 3 August 2018 (but most were published after 2010), in terms of the following qualitative retraction-wise parameters: the main reason for retraction, authorship, a collaboration level, collaborating countries, sources of retraction (a journal or conference proceedings), and funding sources of the research. We also detected journals with high retraction frequencies. Mainly two phrases—“Retraction notice to” and “Retracted Article”—were used to retract publications. The most frequent reason for retractions was plagiarism." (Source: "Analysis of retractions in Indian science" Elango, B., Kozak, M. & Rajendran, P. Scientometrics (2019). First Online: 25 March 2019. https://doi.org/10.1007/s11192-019-03079-y)
Plagiarism (continued)
Plagiarism Euphemisms
- "The other day, we nominated a phrase in a retraction notice for the prize “of most-extra-syllables-used-to-say-the-word-plagiarism” because a journal decided to call the act “inclusion of significant passages of unattributed material from other authors.” (Source: "P values: Scientific journals’ top ten plagiarism euphemisms" Ivan Oransky. Retraction Watch November 29, 2013. emphasis added)
"The Euphemism Parade"
"Why can’t journals just come out and say “plagiarism”?
Science is supposed to be about cold hard truths, about tested hypotheses and convincingly small p values. So why are journal editors so afraid of the p word?
Take these examples of the lengths editors have gone to in order to avoid using the word “plagiarism” in retraction notices:
- From Critical Reviews in Environmental Science and Technology, “contains language from already published sources without using proper citation methods”.
- From the Journal of Biomedical Materials Research Part B: Applied Biomaterials, “unattributed overlap”.
- From International Cardiology, “a duplicate of a paper that has already been published” – by other authors.
- From Landslides, a “significant originality issue”. Can that be remedied by psychoanalysis?
- From Plant Physiology and Biochemistry, “Some sentences…are directly taken from other papers, which could be viewed as a form of plagiarism.” We await word on what else it could be viewed as.
- From the International Journal of Medicine and Biomedical Research, “contains passages from a published article without proper attribution and acknowledgement as if they were original”.
- From Educational Research, an “administrative error”.
- From Chemistry – A European Journal, “the paper was constructed by copying a number of passages from the paper entitled…The authors apologize for this approach.” As we wrote on Retraction Watch, plagiarism is an “approach” to writing the way bank robbery is an approach to banking.
- From Environmental Monitoring and Assessment, “certain parts/portions of the article have been published elsewhere and were not appropriately referenced. The situation is due to honest error…” Aha – so if it is honest, it’s not plagiarism?
- From Rejuvenation Research, “unintended excessive reuse of the text”.
Why use all those words when “plagiarism” will do? Unfortunately, this sort of dancing around the truth is what we see in retraction notices for other kinds of misconduct, too." (Source: "The Euphemism Parade" What’s behind paper retractions? (19) by Adam Marcus and Ivan Oransky, Lab Times 07/2013. Last Changed: 26.11.2013. emphasis added)
"Et Plagieringseventyr" (A Plagiarism Carol), a Norwegian video parody of Charles Dickens' novella A Christmas Carol with English subtitles (Source: University of Bergen (UiB) Published on May 27, 2010. YouTube: https://youtu.be/Mwbw9KF-ACY)
Plagiarism (continued)
"IS IT LIFE-THREATENING TO INVESTIGATE PLAGIARISM?"
"IS IT LIFE-THREATENING TO INVESTIGATE PLAGIARISM?"
- "Yes, it can be. One fresh case.
(...)
"Emilia Șercan is an investigative journalist (for 22 years) working as an assistant professor (for 7 years) at University of Bucharest, Faculty of Journalism and Communication Sciences, Department of Journalism.
(...)
The problem in Romania is that people in all important leadership positions, in all institutions, parties, secret services, administration and even in the academia have plagiarised Ph.D.’s. No one wants a real change. You can’t imagine how hard the battle is here and what kind of obstacles they try to find just not to change things and not to be in position to pay for this,” said E. Șercan.
(...)
She has constantly been a target for intimidation and denigration campaigns, however, she has continued to do her job with respect for the truth, serving solely public interest. The death threat that she received on the evening of 15 April by a SMS message represents yet another attempt to hinder her disclosures."
(...)
On 25 March, E. Șercan revealed that Adrian Iacob, the Rector of the Police Academy, plagiarised more than 70% of his PhD thesis. According to the Romanian law, if a PhD degree is withdrawn for plagiarism, that person can’t be a professor anymore – so Iacob may lose his entire academic career because of her revelations. The police officer who threatened her was not arrested, he was placed under judicial control. The Rector and the Deputy Rector of the Police Academy had been indicted by prosecutors on charges of „instigation to blackmail“ and they have resign. Both are placed under judicial control." (Source: "IS IT LIFE-THREATENING TO INVESTIGATE PLAGIARISM?" Július Kravjar, Member of the board of European Network for Academic Integrity, EuroScientist 24 JUNE, 2019.)
Post-term pregnancy Post-term pregnancy is defined by the World Health Organization and the International Federation of Obstetrics and Gynecology as a pregnancy proceeding to and beyond 294 days of gestation.
Precautionary Principle The Precautionary Principle states if a policy or action has an identified or suspected risk of causing harm to the public or to the environment, in the absence of firm, objective evidence and scientific consensus establishing the policy or action is not harmful, the burden of proof to establish the policy or action is not harmful is the responsibility of those who seek to implement the policy or take the action. (Source: paraphrase of: "The Precautionary Principle (with Application to the Genetic Modification of Organisms)" p. 11. Nassim Nicholas Taleb, Rupert Read, Raphael Douady, Joseph Norman, Yaneer Bar-Yam. EXTREME RISK INITIATIVE —NYU SCHOOL OF ENGINEERING WORKING PAPER SERIES. https://arxiv.org/pdf/1410.5787.pdf and the Wikipedia entry: Precautionary principle)
Precautionary Principle (continued)
The Norwegian National Committee for Research Ethics in Science and Technology
"Guidelines for research ethics in science and technology"
"Uncertainty, risk, and the precautionary principle"
Prolonged pregnancy Prolonged pregnancy is a term used in reference to pregnancies which have progressed to or beyond 287 days of gestation, or 41w+0.
Public interest disclosure
Reproducibility
"Reproducibility of Scientific Results"
"Challenges in irreproducible research"
"Industry is more alarmed about reproducibility than academia"
"Rein in the four horsemen of irreproducibility"
"Dorothy Bishop describes how threats to reproducibility, recognized but unaddressed for decades, might finally be brought under control."
Research Integrity
"Addressing research integrity challenges: from penalising individual perpetrators to fostering research ecosystem quality care"
"The European Code of Conduct for Research Integrity: Revised Edition"
("Europeiske retningslinjer for forskningsintegritet: Revidert Versjon")
ALLEA - All European Academies
Office of Research Integrity: "42 U.S. Code § 289b - Office of Research Integrity"
"Assessing the Integrity of Publicly Funded Research"
Research Integrity (continued)
"Singapore Statement on Research Integrity"
"Preamble
The value and benefits of research are vitally dependent on the integrity of research. While there can be and are national and disciplinary differences in the way research is organized and conducted, there are also principles and professional responsibilities that are fundamental to the integrity of research wherever it is undertaken.
Principles
Responsibilities
Research Integrity (continued)
"Netherlands Code of Conduct for Research Integrity (2018)"
("Nederlandse Gedragscode Wetenschappelijke Integriteit (2018))"
Research Integrity (continued)
"U.S. panel sounds alarm on “detrimental” research practices, calls for new body to help tackle misconduct"
"A new report ["Fostering Integrity in Research"] from the U.S. National Academy of Sciences panel urges the creation of a new, independent group to help tackle research misconduct and other practices that hurt the enterprise.
The report also renames those problematic practices — such as “misleading statistical analysis that falls short of falsification,” awarding authorship to researchers who don’t deserve it (and vice versa), not sharing data, and poorly supervising research — as “detrimental” research practices. In the past, many have dubbed those behaviors as “questionable.”
The reason for the nomenclature change, according to a member of the Committee on Responsible Science (which wrote the report) CK Gunsalus, is to help the community understand that these aren’t just behaviors they should question — they can cause harm. Gunsalus, Director of the National Center for Professional and Research Ethics, told Retraction Watch: "The issue is to raise awareness and get people to think about this seriously, institutions in particular."
The report ["Fostering Integrity in Research"] sticks with the traditional definition of research misconduct — fabrication, falsification, and plagiarism — but emphasizes that those aren’t the only problematic practices, committee chair Robert Nerem said: "Research institutions need to have a much broader focus than fabrication, falsification, and plagiarism — one that includes detrimental research practices — if they are to really foster research integrity."
(...)
Here’s what the report ["Fostering Integrity in Research"] considers to be “detrimental” research practices:
"Fostering Integrity in Research"
"Is it time for a new research integrity board in the U.S.?"
"Overdue: a US advisory board for research integrity"
"Research needs an authoritative forum to hash out collective problems, argue C. K. Gunsalus, Marcia K. McNutt and colleagues."
"Fostering Integrity in Research": FINDINGS AND RECOMMENDATIONS (p. 208-224)
Research Integrity (continued)
"Promoting good scientific practice. The Norwegian case."
"The investigation committee's methods" ("Granskingsutvalgets metoder")
"Make reports of research misconduct public"
"Confronted with bad behaviour, institutions will keep asking the wrong questions until they have to show their [sic] working, says C. K. Gunsalus."
"How to investigate allegations of research misconduct: A checklist"
Precautionary Principle The Precautionary Principle states if a policy or action has an identified or suspected risk of causing harm to the public or to the environment, in the absence of firm, objective evidence and scientific consensus establishing the policy or action is not harmful, the burden of proof to establish the policy or action is not harmful is the responsibility of those who seek to implement the policy or take the action. (Source: paraphrase of: "The Precautionary Principle (with Application to the Genetic Modification of Organisms)" p. 11. Nassim Nicholas Taleb, Rupert Read, Raphael Douady, Joseph Norman, Yaneer Bar-Yam. EXTREME RISK INITIATIVE —NYU SCHOOL OF ENGINEERING WORKING PAPER SERIES. https://arxiv.org/pdf/1410.5787.pdf and the Wikipedia entry: Precautionary principle)
- "The principle implies that there is a social responsibility to protect the public from exposure to harm, when scientific investigation has found a plausible risk. These protections can be relaxed only if further scientific findings emerge that provide sound evidence that no harm will result." (Source: Wikipedia entry: Precautionary principle)
- The Precautionary Principle implies there is a social responsibility to protect women and their fetuses/babies from national medical policies mandating the exclusive use of suboptimal, unilateral ultrasound-based pregnancy dating "methods" implemented within a government-mandated protocol of evidence-obviated medicine; a protocol which is proven to causes sub-optimal obstetric & fetal awareness, which causes suboptimal obstetric & fetal mangement, which causes increased medical risks, critical medical mistakes and grievous medical harms to some of Norway's women and their fetuses/babies. The precautionary principle and evidence-based medicine require the consideration of all available information to ensure the best possible fetal age and gestational age are established for individual pregnancies.
- "9 Researchers must strive to observe the precautionary principle
Where there is plausible, but uncertain knowledge to the effect that a technological application or a development of a research field may lead to ethically unacceptable consequences for health, society, or the environment, the researchers in the field in question must strive to contribute knowledge that is relevant for observing the precautionary principle. This means that researchers must work together with other relevant parties in observing the precautionary principle. The precautionary principle is defined here as follows: "When human activities may lead to morally unacceptable harm that is scientifically plausible but uncertain, actions shall be taken to avoid or diminish that harm." This principle is important for a large part of science and technology research, and researchers have a shared responsibility for ensuring that evaluations are based on the precautionary principle and contribute to avoiding or diminishing harm." (Source: NENT • The National Committee for Research Ethics in Science and Technology, Guidelines for research ethics in science and technology, "Uncertainty, risk, and the precautionary principle" p. 12. View/Download pdf here.) - Primum non nocere, an historical Latin phrase for first, do no harm.
Precautionary Principle (continued)
The Norwegian National Committee for Research Ethics in Science and Technology
"Guidelines for research ethics in science and technology"
"Uncertainty, risk, and the precautionary principle"
- "8 Researchers must clarify the degree of uncertainty in their research and evaluate the risk associated with the research findings
Researchers must clarify the degree of certainty and precision that characterises their research results. They must be particularly meticulous about clarifying the relative certainty and validity range of their findings. In addition to presenting knowledge critically and in context, researchers must strive to point out any risk and uncertainty factors that may have a bearing on the interpretation and possible applications of the research findings. Communicating the relative certainty and validity of knowledge is part of a researcher's ethical responsibility and effort to achieve objectivity. Where possible, researchers should also use appropriate methods for demonstrating the uncertainty of the research. Research institutions have an obligation to teach these methods to their employees and students.
"9 Researchers must strive to observe the precautionary principle
The precautionary principle is defined here as follows: "When human activities may lead to morally unacceptable harm that is scientifically plausible but uncertain, actions shall be taken to avoid or diminish that harm." This principle is important for a large part of science and technology research, and researchers have a shared responsibility for ensuring that evaluations are based on the precautionary principle and contribute to avoiding or diminishing harm." (Source: "Uncertainty, risk, and the precautionary principle" in "Guidelines for research ethics in science and technology" "Issued by The Norwegian National Committee for Research Ethics in Science and Technology (2016). Text: The Norwegian National Committees for Research Ethics, Last updated: Tuesday, June 28, 2016." View/Download pdf here.)
Prolonged pregnancy Prolonged pregnancy is a term used in reference to pregnancies which have progressed to or beyond 287 days of gestation, or 41w+0.
Public interest disclosure
- Public Interest disclosure
"What should you do if you discover research fraud? This is a major ethical dilemma and society has a real need for fraud in research to be reported. The person raising the alarm risks being labelled a whisteblower. In such situations, should you put yourself first and avoid a potentially awkward situation, or should you take responsibility and report the fraud? Or what should you do if your research identifies a potential risk that some people appear to have been exposed to without knowing it? You know that if the risk is real, it may be best to raise the alarm. On the other hand, you also know that there are weaknesses in your research, and that if your research is presented as sensationalist in the media there will be negative consequences." (Source: "Public interest disclosure" The Norwegian National Research Ethics Committees, Arnt Inge Vistnes, Last updated: 17. September 2015) - Public Interest disclosure in cases of dishonesty
"In research we strive for 'intersubjectivity', i.e. research results should, to the greatest extent possible, be independent of the person conducting the research. We believe this will yield results that are 'objective'. One prerequisite for achieving this is that the researchers are honest and that they do not knowingly produce fraudulent data, either by fabricating it or by selecting and retaining only data that matches a particular perception. (See Fraud and plagiarism.)" (Source: ibid.)
Reproducibility
"Reproducibility of Scientific Results"
- "First published Mon Dec 3, 2018
The terms “reproducibility crisis” and “replication crisis” gained currency in conversation and in print over the last decade (e.g., Pashler & Wagenmakers 2012), as disappointing results emerged from large scale reproducibility projects in various medical, life and behavioural sciences (e.g., Open Science Collaboration, OSC 2015). In 2016, a poll conducted by the journal Nature reported that more than half (52%) of scientists surveyed believed science was facing a “replication crisis” (Baker 2016). More recently, some authors have moved to more positive terms for describing this episode in science; for example, Vazire (2018) refers instead to a “credibility revolution” highlighting the improved methods and open science practices it has motivated.
(...)
5. Conclusion
The subject of reproducibility is associated with a turbulent period in contemporary science. This period has called for a re-evaluation of the values, incentives, practices and structures which underpin scientific inquiry. While the meta-science has painted a bleak picture of reproducibility in some fields, it has also inspired a parallel movement to strengthen the foundations of science. However, more progress is to be made, especially in understanding the solutions to the reproducibility crisis. In this regard, there are fruitful avenues for future research, including a deeper exploration of the role that epistemic and non-epistemic values can or should play in scientific inquiry." (Source: "Reproducibility of Scientific Results" Fidler, Fiona and Wilcox, John. The Stanford Encyclopedia of Philosophy, Edward N. Zalta, Editor. The Metaphysics Research Lab, Center for the Study of Language and Information (CSLI), Stanford University. https://plato.stanford.edu/entries/scientific-reproducibility/)
"Challenges in irreproducible research"
- "Science moves forward by corroboration – when researchers verify others’ results. Science advances faster when people waste less time pursuing false leads. No research paper can ever be considered to be the final word, but there are too many that do not stand up to further study.
There is growing alarm about results that cannot be reproduced. Explanations include increased levels of scrutiny, complexity of experiments and statistics, and pressures on researchers. Journals, scientists, institutions and funders all have a part in tackling reproducibility. Nature has taken substantive steps to improve the transparency and robustness in what we publish, and to promote awareness within the scientific community. We hope that the articles contained in this collection will help." (Source: "Challenges in irreproducible research" Nature Special | 18 October 2018.)
"Industry is more alarmed about reproducibility than academia"
- "The reproducibility crisis in biomedical science seems to have alarmed industry more than the academic community (see C. G. Begley and L. M. Ellis Nature 483, 531–533; 2012). In our view, this is because they have different yardsticks for success in research. Despite the advent of important new therapeutics, the number of innovative treatments reaching the patient is disappointingly low. To help rectify this, industry is investing in drug-discovery alliances with peers and academic groups, and in precision medicine. It sees high standards of research quality as the route to the most promising drug candidates and to maximum return on investment.
By contrast, academic scientists may be reluctant to devote extra time and effort to confirming research results in case they fail. That would put paid to publication in high-impact journals, damage career opportunities and curtail further funding. Evidence of questionable practices such as selective publishing and cherry-picking of data indicates that rigour is not always a high priority.
Paradoxically, the impact of high standards on research objectives is different in industry and in academia. If ignored, this paradox could endanger future collaborations between scientists in the private and public sectors." (Source: "Industry is more alarmed about reproducibility than academia" CORRESPONDENCE 28 NOVEMBER 2018 Nature 563, 626 (2018) doi: 10.1038/d41586-018-07549-w)
"Rein in the four horsemen of irreproducibility"
"Dorothy Bishop describes how threats to reproducibility, recognized but unaddressed for decades, might finally be brought under control."
- "By contrast, I think that, in two decades, we will look back on the past 60 years — particularly in biomedical science — and marvel at how much time and money has been wasted on flawed research.
(...)
Yet many researchers persist in working in a way almost guaranteed not to deliver meaningful results. They ride with what I refer to as the four horsemen of the reproducibility apocalypse: publication bias, low statistical power, P-value hacking and HARKing (hypothesizing after results are known). My generation and the one before us have done little to rein these in." (Source: "Rein in the four horsemen of irreproducibility" Dorothy Bishop. Nature 568, 435 (2019) 24 APRIL 2019 doi: 10.1038/d41586-019-01307-2)
Research Integrity
"Addressing research integrity challenges: from penalising individual perpetrators to fostering research ecosystem quality care"
- "Introduction
Concern for and interest in research integrity has increased significantly during recent decades, both in academic and in policy discourse (Horbach & Halffman 2017). Notably in the public realm, integrity debates are often triggered by spectacular (high visibility) misconduct cases, committed by prominent scientists (or even science celebrities), such as the Schön case (Consoli 2006), the Hwang case (Gottweis & Triendl 2006; Zwart 2008), the Macchiarini case (Vogel 2016) and the Stapel case (Zwart 2017), conveying a common narrative structure, starting with a spectacular ascent, based on fraud, and resulting in a dramatic fall from grace and followed by an avalanche of academic and public comments. Such cases fuel the question how widespread (or even endemic) misconduct practices in contemporary research have become, and how the current wave of integrity challenges in contemporary research can best be addressed. This editorial to the LSSP thematic series (article collection) entitled Addressing integrity challenges in research: the institutional dimension invites authors to contribute to the research integrity debate." (Source: "Addressing research integrity challenges: from penalising individual perpetrators to fostering research ecosystem quality care" Zwart and Meulen. Life Sciences, Society and Policy (2019) 15:5 https://doi.org/10.1186/s40504-019-0093-6)
"The European Code of Conduct for Research Integrity: Revised Edition"
("Europeiske retningslinjer for forskningsintegritet: Revidert Versjon")
ALLEA - All European Academies
- "3. Violations of Research Integrity
It is of crucial importance that researchers master the knowledge, methodologies and ethical practices associated with their field. Failing to follow good research practices violates professional responsibilities. It damages the research processes, degrades relationships among researchers, undermines trust in and the credibility of research, wastes resources and may expose research subjects, users, society or the environment to unnecessary harm.
3.1 Research Misconduct and other Unacceptable Practices
Research misconduct is traditionally defined as fabrication, falsification, or plagiarism (the so-called FFP categorisation) in proposing, performing, or reviewing research, or in reporting research results:
• Fabrication is making up results and recording them as if they were real.
• Falsification is manipulating research materials, equipment or processes or changing, omitting or suppressing data or results without justification.
• Plagiarism is using other people’s work and ideas without giving proper credit to the original source, thus violating the rights of the original author(s) to their intellectual outputs.
These three forms of violation are considered particularly serious since they distort the research record. There are further violations of good research practice that damage the integrity of the research process or of researchers. In addition to direct violations of the good research practices set out in this Code of Conduct, examples of other unacceptable practices include, but are not confined to:
• Manipulating authorship or denigrating the role of other researchers in publications.
• Re-publishing substantive parts of one’s own earlier publications, including translations, without duly acknowledging or citing the original (‘self-plagiarism’).
• Citing selectively to enhance own findings or to please editors, reviewers or colleagues.
• Withholding research results.
• Allowing funders/sponsors to jeopardise independence in the research process or reporting of results so as to introduce or promulgate bias.
• Expanding unnecessarily the bibliography of a study.
• Accusing a researcher of misconduct or other violations in a malicious way.
• Misrepresenting research achievements.
• Exaggerating the importance and practical applicability of findings.
• Delaying or inappropriately hampering the work of other researchers.
• Misusing seniority to encourage violations of research integrity.
• Ignoring putative violations of research integrity by others or covering up inappropriate responses to misconduct or other violations by institutions.
• Establishing or supporting journals that undermine the quality control of research (‘predatory journals’).
Office of Research Integrity: "42 U.S. Code § 289b - Office of Research Integrity"
- (a) In general
(1) Establishment of Office
Not later than 90 days after June 10, 1993, the Secretary shall establish an office to be known as the Office of Research Integrity (referred to in this section as the “Office”), which shall be established as an independent entity in the Department of Health and Human Services." (Source: Cornell University, Cornell Law School, Legal Information Institute (LII) "42 U.S. Code § 289b - Office of Research Integrity")
"Assessing the Integrity of Publicly Funded Research"
- Ingegrity
"Integrity is a measure of wholeness or completeness. When applied to professional behavior, it is essentially a measure of the degree to which someone’s (or some institution’s) actions accord with ideal or expected behavior. However, the ideals or expected behaviors for professional conduct are complex, not always well defined, and subject to change or reinterpretation. I have, therefore, adopted a fairly inclusive definition of integrity and assumed that it can be thought of as a measure of the degree to which researchers adhere to the rules or laws, regulations, guidelines, and commonly accepted professional codes and norms of their respective research areas." (Source: "Assessing the Integrity of Publicly Funded Research: A Background Report for the November 2000 ORI Research Conference on Research Integrity" Nicholas H. Steneck, Ph.D. University of Michigan, ORI Consultant. November 2000. p. 2)
"Bias and Conflict of Interest
"There has been considerable debate about the role of values and personal interest in research ever since Merton proposed “disinterestedness” as one of four key values on which science rests (Merton 1942, p. 116). It is now widely recognized that values influence research (Jasanoff 1996), but there is also a common understanding that the influence of values should be minimized and made public, particularly when financial interests are involved.
Considerable evidence exists to support the contention that personal interest does influence research behavior. Positive-outcome bias (favoring publications that report positive results over those that report negative results or that do not find results) has been demonstrated in a number of studies (Mahoney 1977; Dickersin 1992; Callaham 1998). The reverse effect has also been reported, that is, slower publication rates for studies that fail to find a particular result (Misakian 1998). Studies are just beginning to assess how these interests affect research and whether they are being properly managed (Campbell 1999; Boyd 2000; Cho 2000)
In calling controversial publication, reporting, and other research practices “questionable,” the NAS report, Responsible Science,” highlighted an important problem. “Integrity” is not an all-or-nothing proposition. There is a difference between a failure to check the spelling of every author’s name or to catch every typo and using improper statistics or delaying the publication of a manuscript to please a sponsor. It is not easy to pinpoint where or when high standards for integrity in research give way to careless research practices, to irresponsible research practices or to misconduct. The extremes (high standards for integrity and misconduct) can be defined, but behaviors that fall between, to one extent or another, are all subject to interpretation. This, in turn, makes it imperative that these behaviors are well understood and their consequences evaluated, both as part of the process of reassuring the public that its research funds are being spent responsibility and as needed background information for developing responsible conduct of research training programs." (Source: ibid., p. 11-12)
Significance of Misconduct Matters Most
"The results of surveys, audits, and estimates of the rate of under-reporting raise two important issues for further consideration. First, however the results of surveys and audits are ultimately interpreted or clarified, there remains the troubling discrepancy between public statements about how “rare” misconduct in research supposedly is and the more private belief on the part of many researchers that it is in fact fairly common. How can these two views be reconciled?
Second, whatever the actual rate of misconduct, it is not so much the rate as the significance of the misconduct that matters most. Summarizing the results of scientific data audits of the Cancer and Leukemia Group B’s clinical trials, Weiss et al. conclude that “scientific improprieties have occurred very rarely...” (Weiss 1993, p. 459). “Very rarely,” in this case, is based on a quantitative estimate of 0.28% (p. 462)–28 cases of misconduct for every 10,000 clinical researchers or one case for every 357 clinical researchers. On what basis can this rate be judged as either “rare” or “significant”? Clearly, understanding the importance of misconduct in research requires not only better estimates of numbers but also of significance. How much does a case of misconduct in research actually cost the public in terms of wasted research dollars, of deceptive findings that mislead other researchers until the misconduct is discovered, and perhaps of negative impacts on patient health?" (Source: ibid., p. 3-4)
Research Integrity (continued)
"Singapore Statement on Research Integrity"
"Preamble
The value and benefits of research are vitally dependent on the integrity of research. While there can be and are national and disciplinary differences in the way research is organized and conducted, there are also principles and professional responsibilities that are fundamental to the integrity of research wherever it is undertaken.
Principles
- Honesty in all aspects of research
- Accountability in the conduct of research
- Professional courtesy and fairness in working with others
- Good stewardship of research on behalf of others
Responsibilities
- Integrity: Researchers should take responsibility for the trustworthiness of their research.
- Adherence to Regulations: Researchers should be aware of and adhere to regulations and policies related to research.
- Research Methods: Researchers should employ appropriate research methods, base conclusions on critical analysis of the evidence, and report findings and interpretations fully and objectively.
- Research Records: Researchers should keep clear, accurate records of all research in ways that will allow verification and replication of their work by others.
- Research Findings: Researchers should share data and findings openly and promptly, as soon as they have had an opportunity to establish priority and ownership claims.
- Authorship: Researchers should take responsibility for their contributions to all publications, funding applications, reports, and other representations of their research. Lists of authors should include all those and only those who meet applicable authorship criteria.
- Publication Acknowledgment: Researchers should acknowledge in publications the names and roles of those who made significant contributions to the research, including writers, funders, sponsors, and others, but do not meet authorship criteria.
- Peer Review: Researchers should provide fair, prompt, and rigorous evaluations and respect confidentiality when reviewing others’ work.
- Conflict of Interest: Researchers should disclose financial and other conflicts of interest that could compromise the trustworthiness of their work in research proposals, publications, and public communications as well as in all review activities.
- Public Communication: Researchers should limit professional comments to their recognized expertise when engaged in public discussions about the application and importance of research findings and clearly distinguish professional comments from opinions based on personal views.
- Reporting Irresponsible Research Practices: Researchers should report to the appropriate authorities any suspected research misconduct, including fabrication, falsification, or plagiarism, and other irresponsible research practices that undermine the trustworthiness of research, such as carelessness, improperly listing authors, failing to report conflicting data, or the use of misleading analytical methods.
- Responding to Irresponsible Research Practices: Research institutions, as well as journals, professional organizations, and agencies that have commitments to research, should have procedures for responding to allegations of misconduct and other irresponsible research practices and for protecting those who report such behavior in good faith. When misconduct or other irresponsible research practice is confirmed, appropriate actions should be taken promptly, including correcting the research record.
- Research Environments: Research institutions should create and sustain environments that encourage integrity through education, clear policies, and reasonable standards for advancement, while fostering work environments that support research integrity.
- Societal Considerations: Researchers and research institutions should recognize that they have an ethical obligation to weigh societal benefits against risks inherent in their work."
(Source: "The Singapore Statement on Research Integrity" NIH Public Access, Author Manuscript. David B. Resnik, J.D., Ph.D. and Adil E. Shamoo, Ph.D. Published in final edited form as: Account Res. 2011 March ; 18(2): 71–75. doi:10.1080/08989621.2011.557296.)
Research Integrity (continued)
"Netherlands Code of Conduct for Research Integrity (2018)"
("Nederlandse Gedragscode Wetenschappelijke Integriteit (2018))"
Research Integrity (continued)
"U.S. panel sounds alarm on “detrimental” research practices, calls for new body to help tackle misconduct"
"A new report ["Fostering Integrity in Research"] from the U.S. National Academy of Sciences panel urges the creation of a new, independent group to help tackle research misconduct and other practices that hurt the enterprise.
The report also renames those problematic practices — such as “misleading statistical analysis that falls short of falsification,” awarding authorship to researchers who don’t deserve it (and vice versa), not sharing data, and poorly supervising research — as “detrimental” research practices. In the past, many have dubbed those behaviors as “questionable.”
The reason for the nomenclature change, according to a member of the Committee on Responsible Science (which wrote the report) CK Gunsalus, is to help the community understand that these aren’t just behaviors they should question — they can cause harm. Gunsalus, Director of the National Center for Professional and Research Ethics, told Retraction Watch: "The issue is to raise awareness and get people to think about this seriously, institutions in particular."
The report ["Fostering Integrity in Research"] sticks with the traditional definition of research misconduct — fabrication, falsification, and plagiarism — but emphasizes that those aren’t the only problematic practices, committee chair Robert Nerem said: "Research institutions need to have a much broader focus than fabrication, falsification, and plagiarism — one that includes detrimental research practices — if they are to really foster research integrity."
(...)
Here’s what the report ["Fostering Integrity in Research"] considers to be “detrimental” research practices:
- -Detrimental authorship practices that may not be considered misconduct, such as honorary authorship, demanding authorship in return for access to previously collected data or materials, or denying authorship to those who deserve to be designated as authors.
- -Not retaining or making data, code, or other information/materials underlying research results available as specified in institutional or sponsor policies, or standard practices in the field.
- -Neglectful or exploitative supervision in research.
- -Misleading statistical analysis that falls short of falsification.
- -Inadequate institutional policies, procedures, or capacity to foster research integrity and address research misconduct allegations, and deficient implementation of policies and procedures.
- -Abusive or irresponsible publication practices by journal editors and peer reviewers."
(Source: "U.S. panel sounds alarm on “detrimental” research practices, calls for new body to help tackle misconduct" Alison McCook. Retraction Watch April 11, 2017)
"Fostering Integrity in Research"
- “In recent years, as ongoing globalization, technological advances, and other shifts have transformed research, it is clear that the research enterprise faces new and complex challenges in fostering integrity and in dealing with the consequences of research misconduct and detrimental research practices. Serious cases of research misconduct—including some that have gone undetected for years—continue to emerge with disturbing regularity in the United States and around the world. Increases in the number and percentage of research articles that are retracted and growing concern about low rates of reproducibility in some research fields raise questions about how the research enterprise can better ensure that investments in research produce reliable knowledge.
It is necessary for all of us involved in performing, managing, funding, and communicating research to commit to improving practices in our own organizations and disciplines as well as more broadly. Key areas of focus include institutional efforts to sustain research environments conducive to integrity, greatly expanded sharing of data and code, more complete reporting of results, more responsible approaches to scholarly publishing, better understanding of the causes and consequences of breaches in integrity, and clearer standards for authorship. While this report provides a framework and rationale for positive change, collective action on the part of the community will be necessary to push forward toward a research future characterized by greater integrity and quality. (...) –Robert M. Nerem, Chair, Committee on Responsible Science" (Source: "Fostering Integrity in Research" A Consensus Study Report of The National Academies of Sciences, Engineering and Medicine. 2017. Washington, DC: The National Academies Press. doi: https://doi.org/10.17226/21896 Preface ix-x)
- "THE TRANSLATIONAL OMICS CASE AT DUKE
Synopsis and Rationale for Inclusion: The case of Duke University researchers Joseph Nevins and Anil Potti, which stretched out over several years and attracted national media attention, illustrates shortcomings and deficiencies in current approaches to research integrity on the part of researchers, research institutions, government agencies and journals (CBS News, 2012). Potti's fabricated results endangered trial participants and may have contributed to public mistrust in scientific research. Institutionally, supervisors at the laboratory level and senior administrators did not respond effectively for several years despite multiple warning signs. This case also raises questions about the responsibility of a journal to respond appropriately if numerous inquiries are made on the same original article. Several parties' unresponsiveness to questions on Potti's work may have delayed the findings of research misconduct." (Source: Ibid., Appendix D, p. 274; or see: https://www.ncbi.nlm.nih.gov/books/NBK475955/) - "Abstract
Academic medical centers rarely require all of their research faculty and staff to participate in educational programs on the responsible conduct of research (RCR). There is also little published evidence of RCR programs addressing high-profile, internal cases of misconduct as a way of promoting deliberation and learning. In the wake of major research misconduct, Duke University School of Medicine (DUSoM) expanded its RCR education activities to include all DUSoM faculty and staff engaged in research. The program included formal deliberation of the Translational Omics misconduct case, which occurred at Duke. Over 5,000 DUSoM faculty and staff participated in the first phase of this new program, with a 100% completion rate. The article reports on the program’s development, challenges and successes, and future directions. This experience at Duke University illustrates that, although challenging and resource intensive, engagement with RCR activities can be integrated into programs for all research faculty and staff. Formal, participatory deliberation of recent cases of internal misconduct can add a novel dimension of reflection and openness to RCR educational activities." (Source: "Implementation of a responsible conduct of research education program at Duke University School of Medicine" Christian Simon, Rebecca W. Beerman, Jennifer L. Ariansen, Donna Kessler, Ana M. Sanchez, Kindra King, Marcella Sarzotti-Kelsoe, Gregory Samsa, Ann Bradley, Laurianne Torres, Robert Califf & Geeta K. Swamy. Accountability in Research Policies and Quality Assurance Volume 26, 2019 - Issue 5. https://doi.org/10.1080/08989621.2019.1621755)
"Is it time for a new research integrity board in the U.S.?"
- "Nearly two years ago, a report from the U.S. National Academies of Science, Engineering and Medicine (NASEM) called for a new advisory board that would promote research integrity and tackle misconduct. That board does not yet exist, but today in Nature, five authors, led by C. K. Gunsalus of the University of Illinois at Urbana-Champaign, argue that it should, and describe next steps in its creation. We asked Gunsalus a few questions about the idea."
(...)
"RW [Retraction Watch]: You point out that experts — including yourself and two of your co-authors — have been recommending such a board since 1992. Why hasn’t the idea taken hold, and do you think that 2019 is different?
CKG [C. K. Gunsalus]: People are busy and the world keeps moving. Structural responses tend to be event- and scandal-driven, so attention waxes and wanes depending on the events of the moment. What has changed since 1992 is that these problems have not gone away. The work of Retraction Watch and many others has demonstrated that there are recurring problems for which we do not have in place systems that are as effective as the research community–and those affected by the research we conduct–needs." (Source: "Is it time for a new research integrity board in the U.S.?" Ivan Oransky. Retraction Watch February 11, 2019)
"Overdue: a US advisory board for research integrity"
"Research needs an authoritative forum to hash out collective problems, argue C. K. Gunsalus, Marcia K. McNutt and colleagues."
- "Building a culture of quality and integrity requires conversations across the scientific enterprise. Science is a complex ecosystem of funders, journals, academic administrators, scientific societies and researchers — the latter group including principal investigators, staff scientists, postdocs and graduate students. The interests of each group conflict as often as they overlap, and interactions tend to be stratified and constrained. Institutional presidents sit on working groups with each other but not with research-integrity officers. These officers attend conferences with each other, but not with faculty advisers and bench scientists. Journal editors meet scientists and other editors, but not institutional officers, on whom they rely for investigation when concerns about manuscripts arise." (Source: "Overdue: a US advisory board for research integrity" C. K. Gunsalus, Marcia K. McNutt, Brian C. Martinson, Larry R. Faulkner & Robert M. Nerem. Nature 566, 173-175 (2019) doi: 10.1038/d41586-019-00519-w)
"Fostering Integrity in Research": FINDINGS AND RECOMMENDATIONS (p. 208-224)
- RECOMMENDATION ONE: In order to better align the realities of research with its values and ideals, all stakeholders in the research enterprise—researchers, research institutions, research sponsors, journals, and societies—should significantly improve and update their practices and policies to respond to the threats to research integrity identified in this report.
- RECOMMENDATION TWO: Since research institutions play a central role in fostering research integrity and addressing current threats, they should maintain the highest standards for research conduct, going beyond simple compliance with federal regulations in undertaking research misconduct investigations and in other areas.
- RECOMMENDATION THREE: Research institutions and federal agencies should work to ensure that good-faith whistleblowers are protected and that their concerns are assessed and addressed in a fair, thorough, and timely manner.
- RECOMMENDATION FOUR: To provide a continuing organizational focus for fostering research integrity that cuts across disciplines and sectors, a Research Integrity Advisory Board (RIAB) should be established as an independent nonprofit organization. The RIAB will work with all stakeholders in the research enterprise—researchers, research institutions, research sponsors and regulators, journals, and scientific societies—to share expertise and approaches for addressing and minimizing research misconduct and detrimental research practices. The RIAB will also foster research integrity by stimulating efforts to assess research environments and to improve practices and standards.
- RECOMMENDATION FIVE: Societies and journals should develop clear disciplinary authorship standards. Standards should be based on the principle that those who have made a significant intellectual contribution are authors. Significant intellectual contributions can be made in the design or conceptualization of a study, the conduct of research, the analysis or interpretation of data, or the drafting or revising of a manuscript for intellectual content. Those who engage in these activities should be designated as authors of the reported work, and all authors should approve the final manuscript. In addition to specifying all authors, standards should (1) provide for the identification of one or more authors who assume responsibility for the entire work, (2) require disclosure of all author roles and contributions, and (3) specify that gift or honorary authorship, coercive authorship, ghost authorship, and omitting authors who have met the articulated standards are always unacceptable. Societies and journals should work expeditiously to develop such standards in disciplines that do not already have them.
- RECOMMENDATION SIX: Through their policies and through the development of supporting infrastructure, research sponsors and science, engineering, technology, and medical journal and book publishers should ensure that information sufficient for a person knowledgeable about the field and its techniques to reproduce reported results is made available at the time of publication or as soon as possible after that.
- RECOMMENDATION SEVEN: Federal funding agencies and other research sponsors should allocate sufficient funds to enable the long-term storage, archiving, and access of datasets and code necessary for the replication of published findings.
- RECOMMENDATION EIGHT: To avoid unproductive duplication of research and to permit effective judgments on the statistical significance of findings, researchers should routinely disclose all statistical tests carried out, including negative findings. Research sponsors, research institutions, and journals should support and encourage this level of transparency.
- RECOMMENDATION NINE: Government agencies and private foundations that support science, engineering, and medical research in the United States should fund research to quantify, and develop responses to, conditions in the research environment that may be linked to research misconduct and detrimental research practices. These research sponsors should use the data accumulated to monitor and modify existing policies and regulations.
- RECOMMENDATION TEN: Researchers, research sponsors, and research institutions should continue to develop and assess more effective education and other programs that support the integrity of research. These improved programs should be widely adopted across disciplines and across national borders.
- RECOMMENDATION ELEVEN: Researchers, research institutions, and research sponsors that participate in and support international collaborations should leverage these partnerships to foster research integrity through mutual learning and sharing of best practices, including collaborative international research on research integrity.
(Source: "Fostering Integrity in Research" A Consensus Study Report of The National Academies of Sciences, Engineering and Medicine. 2017. Washington, DC: The National Academies Press. doi: https://doi.org/10.17226/21896)
Research Integrity (continued)
"Promoting good scientific practice. The Norwegian case."
- "What does the Norwegian National Research Ethics Committees do to promote good scientific practices/research integrity?
A lot of things. We have Committees for different fields of research; they are developing guidelines, which are also codes for good research practice, since the early 90s. Our system has not been so much active in investigating research misconduct, as in many other countries. It is based on building up good practice and establish a culture of good scientific practice." (Source: "Promoting good scientific practice. The Norwegian case." Interview with Annette Birkeland, attorney for the Norwegian National Commission for the Investigation of Research Misconduct, financed and administered by the Ministry of Education and Research", headed by; Minister of Research and Higher Education). Elephant in the Lab, 18 June 2018 | doi:10.5281/zenodo.1251664)
"The investigation committee's methods" ("Granskingsutvalgets metoder")
- "Our research ethics system is based on the idea of academic self-government. Therefore, it is primarily the institutions themselves who are to deal with cases of possible misconduct. When cases are solved at the lowest possible level, the processing time is also shortest. And it is the institutions themselves who have the ability to respond to misconduct. Most of the misconduct cases are dealt with internally by the research institutions, and in several cases the conclusion has been that researchers have acted scientifically unfairly."
"Vårt forskningsetiske system bygger på tanken om akademisk selvstyre. Derfor er det også først og fremst institusjonene selv som skal behandle saker om mulig uredelighet. Når saker løses på lavest mulig nivå, blir også behandlingstiden kortest. Og det er institusjonene selv som har reaksjonsmuligheter ved overtramp. De fleste uredelighetssakene behandles internt ved forskningsinstitusjonene, og i flere saker har konklusjonen vært at forskere har opptrådt vitenskapelig uredelig." (Source: "Granskingsutvalgets metoder" Av jurist Annette Birkeland og kommunikasjonsrådgiver Ingrid S. Torp, De nasjonale forskningsetiske komiteene. Sist oppdatert: 28. juni 2017)
- A real-world example of Norway's naive "idea of academic self-government" for allegations of research misconduct follows.
"Meeting of the Investigation Committee on 22 November 2018" ("Møte i Granskingsutvalget 22. november 2018")
Public protocol from the meeting of the National Committee for Investigation of Misconduct in Research (the Investigation Committee). ("Offentlig protokoll fra møte i Nasjonalt utvalg for gransking av uredelighet i forskning (Granskingsutvalget)")
(...)
Item 5: Discussion Cases (Sak 5: Diskusjonssaker)
(...)
"C. Case no. 2018/308 - Inquiry about possible plagiarism of idea / method / algorithm
As a general rule, inquiries regarding possible breaches of recognized research ethical standards shall be dealt with by the research institution in which the investigated researcher is employed, cf. Section 6 of the Research Ethics Act, § 6. The investigation committee does not find grounds for departing from the main rule of the Act.
("C. Saksnr. 2018/308 – Henvendelse om mulig plagiat av idé/metode/algoritme
Henvendelser om mulige brudd på anerkjente forskningsetiske normer skal som hovedregel behandles av forskningsinstitusjonen der den innklagede forskeren er ansatt, jf. forskningsetikkloven § 6. Granskingsutvalget finner ikke grunnlag for å fravike lovens hovedregel.)
The Investigation Committee's decision: The inquiry is sent to the University of Oslo for processing, cf. Section 6, first paragraph, of the Research Ethics Act"
(Granskingsutvalgets beslutning: Henvendelsen oversendes Universitetet i Oslo for behandling, jf. forskningsetikkloven § 6 første ledd.") (Source: "Meeting of the Investigation Committee on 22 November 2018" ("Møte i Granskingsutvalget 22. november 2018") Tid: 1000 - 1430; Sted: Kongens gate 14, Oslo; Til stede: Ragna Aarli, Jan G. Bjålie, Rani Lill Anjum, Per-Henrik Zahl, Anne I. Myhr, Nina Johannesen, Øyvind K. Arnesen, Arild Underdal, John-Arne Skolbekken, Ida Skaar; Forfall: Lise Wogensen Bach; Fra sekretariatet: Annette Birkeland (referent)) [Note: The 1st paragraph of Section 6 of the Research Ethics Act states: "Research institutions are responsible for dealing with cases of possible breaches of recognized ethical standards of research ethics. Chapter IV and V of the Public Administration Act apply to these matters."]
"Make reports of research misconduct public"
"Confronted with bad behaviour, institutions will keep asking the wrong questions until they have to show their [sic] working, says C. K. Gunsalus."
- "Even when investigations are exemplary and findings clear, universities rarely report them publicly. That secrecy perpetuates misbehaviour and breeds mistrust..." (Source: "Make reports of research misconduct public" C. K. Gunsalus (Director of the National Center for Professional and Research Ethics at the University of Illinois Urbana–Champaign. e-mail: [email protected]). Nature VOL 570, 6 JUNE 2019. p.7) [Also see: "“Our current approaches are not working:” Time to make misconduct investigation reports public, says integrity expert" Ivan Oransky. Retraction Watch. June 4, 2019)
- "Science is fast becoming more transparent. So, too, should institutional practice. Open misconduct reports would create a virtuous circle. Institutions would learn from their own and others’ investigations. Leaders would be more likely to pay attention to reports that are subject to scrutiny. Honest researchers could see that although groundbreaking science is often uncertain, it is qualitatively different from the conduct that leads to misconduct reviews." (Source: Ibid.)
- "It’s time to do better. Misconduct investigations will be more effective and potentially even lead to systemic improvements if they are framed in the right way, with questions, such as: ‘Do you want your reputation associated with an institution that countenances dishonest work?’, ‘How might this have been prevented?’, ‘What are our students learning about how to do research?’ and ‘Are other scholars depending on this work?’ Institutional procedures must not only protect the rights of whistle-blowers and those accused, but also protect students, colleagues, scholars and the advancement of knowledge. I propose a three-part approach: a checklist to strengthen investigations; the external peer review of investigatory reports; and the publication of findings." (Source: Ibid.) [See: "Institutional Research Misconduct Reports Need More Credibility" C. K. Gunsalus, JD; Adam R. Marcus, MA; Ivan Oransky, MD. JAMA. 2018;319(13):1315-1316. doi:10.1001/jama.2018.0358]
- "Even the best guidelines are of limited value without a mechanism for holding investigators accountable. We use peer review for research; we should do so for investigations, too. Consortia of universities could work together to peer review and certify reports before they are finalized. " (Source: Ibid.) [See: "Peer Review Form for Research Integrity Investigation Reports" National Center for Professional Research Ethics (NCPRE); Retraction Watch (RW)]
"How to investigate allegations of research misconduct: A checklist"
- "In preparing The List, we referred to “the Guidelines for Responding to Misconduct in Research” from the Japanese Ministry of Education, Culture, Sports, Science and Technology (effective August 26, 2014) and the article, “Institutional research misconduct reports need more credibility” (Gunsalus et al. JAMA 319: 1315-1316, 2018, published as a statement of the Expert Meeting held in Chicago, Illinois, U.S. in December 2017). This article presents a “Peer Review Form for Research Integrity Investigation Reports” which lists key aspects of investigation reports for quality evaluation. In contrast, The List itemizes critical issues that must be considered when initiating, conducting and reporting the results of investigations." (Source: "How to investigate allegations of research misconduct: A checklist" Iekuni Ichikawa. Retraction Watch January 8, 2019) and (Source: "The List" or "Checklist for Investigating Allegations of Research Misconduct" Association for the Promotion of Research Integrity (APRIN), Japan)
Research Misconduct
Promoting Integrity as an Integral Dimension of Excellence in Research
"Final Report on the Incidence of Misconduct"
Promoting Integrity as an Integral Dimension of Excellence in Research
"Final Report on the Incidence of Misconduct"
- "3 Norway
3.1 Research misconduct and its registration in Norway
On the national level in Norway, the handling of research misconduct accusation is primarily regulated through The Act Relating to Ethics and Integrity in Research (Research Ethics Act) 12. Even though there are regulations on the national level, cases are mostly handled on the local level, by the institutions themselves. The institutions have a high degree of autonomy when it comes to handling such cases, and do their own investigations. They are also responsible for punishing any misconduct they uncover.
This autonomy has contributed to a high diversity of practices. Some institutions have ethics committees that handle misconduct cases, while others handle such cases ad hoc. The institutions are not required to report accusations of misconduct locally or to any central institution, and information about misconduct cases is therefore not readily available. In one instance, the Norwegian University for Science and Technology, Norway’s largest university, the responsibility is decentralized even further. This institution reported that handling misconduct is the responsibility of the different faculties, and that the central/top level therefore had little knowledge about the universities’ practices or instances of misconduct.
Norway has a National Commission for the Investigation of Research Misconduct 13. Its mandate is to be a national resource for institutions and private companies in cases of misconduct, and to supplement institutions in the handling of such cases. This commission handles some cases, mostly by request from the institutions, when the case is especially hard, there are conflicts of interest or the case has gotten a lot of public attention. The commission can also handle cases on its own initiative, but it is not in its mandate to sanction those they find to be guilty of misconduct. That responsibility rests on the institutions, and it is up to them to decide what to do with the commission’s conclusions. As the Commission only has a supplementary role, they send most of the cases that is reported to them back to the institution where the alleged misconduct took place." (Source: "Promoting Integrity as an Integral Dimension of Excellence in Research: "Final Report on the Incidence of Misconduct" Marijke Van Buggenhout , Jenneke Christiaens & Serge Gutwirth (Vrije Universiteit Brussel - VUB) PRINTEGER. p. 38-39. Actual Submission Date Month 21, 30/05/2017)
Research Misconduct
"They did a bad bad thing"
When it comes to research misconduct, burying one's head in the sand and pretending it doesn't exist is the worst possible plan.
Department of Health and Human Service, Office of Research Integrity
Research Misconduct (continued)
"The European Code of Conduct for Research Integrity: Revised Edition"
("Europeiske retningslinjer for forskningsintegritet: REVIDERT VERSJON")
ALLEA - All European Academies
Research Misconduct (continued)
"CHECKLIST: Policies and Procedures for Handling Research Misconduct Allegations"
"This checklist is used by the U.S. Department of Health and Human Services, Office of Research Integrity (ORI) and is intended only to provide general information regarding ORI’s review of institutional policies. " (Source: "CHECKLIST: Policies and Procedures for Handling Research Misconduct Allegations" Last Modified: 04/05/2017. U.S. Office of Research Integrity
"How to investigate allegations of research misconduct: A checklist"
"Do investigations into research misconduct allegations need better standards? The Association for the Promotion of Research Integrity (APRIN) in Japan, a group of volunteers who “commit themselves to the promotion of research of high integrity” and provide “e-learning material for research ethics education,” thinks so. Today, we present a guest post by Iekuni Ichikawa, who chaired an APRIN committee that recently came up with a new checklist for such investigations, about the effort. (Source: "How to investigate allegations of research misconduct: A checklist" Iekuni Ichikawa. Retraction Watch Posted on January 8, 2019; Also see: "Checklist for Investigating Allegations of Research Misconduct")
"Investigation of Allegations of Research Misconduct"
"Research misconduct is very narrowly defined as: (1) Fabrication of results, making up data; (2) Falsification of data, manipulating research materials, equipment or processes, or changing or omitting data or results such that the research is not accurately represented in the research record or; (3) Plagiarism, the appropriation of another person’s ideas, processes, results or words without giving credit.
Maintaining the highest ethical standards in the conduct of research remains the goal of the entire scientific community at the NIH. Our good reputation is essential for maintaining the trust of Congress, the American People, our Scientific Colleagues across the US and the International Community. It is the responsibility of each Investigator who participates in research at the NIH, no matter what their career stage, to maintain exemplary standard of intellectual honesty in formulating or conducting research and to report any suspected research misconduct to the NIH Agency Research Integrity Officer (ARIO)."
Research Misconduct (continued)
U.S. National Science Foundation (NSF)
Included below is the formal definition of research misconduct by the U.S. National Science Foundation (NSF), the largest funding organization of research and education in most fields of science and engineering.
(a) Research misconduct means fabrication, falsification, or plagiarism in proposing or performing research funded by NSF, reviewing research proposals submitted to NSF, or in reporting research results funded by NSF.
Research Misconduct (continued)
U.S. Federal Register, Part III, Department of Health and Human Services
CFR › Title 42 › Chapter I › Subchapter H › Part 93 › Subpart C › Section 93.310
"§ 93.310 Institutional investigation.
Institutions conducting research misconduct investigations must:
§ 93.103 Research misconduct.
Research misconduct means fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
Research Misconduct (continued)
Stanford University
Research Policy Handbook
1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting
3. Definitions
"A. Research Misconduct
"Research misconduct" is defined as fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
A finding of research misconduct requires that:
"Understanding Stanford’s Code of Conduct"
Research Misconduct (continued)
The International Association of Scientific, Technical and Medical Publishers (STM)
"The STM Report: An overview of scientific and scholarly publishing, Fifth edition, October 2018"
"Scientific Misconduct and Medical Journals"
"Duke University to settle case alleging researchers used fraudulent data to win millions in grants"
"It’s time to end the code of silence at universities"
"The association between quality measures of medical university press releases and their corresponding news stories—Important information missing"
"Bulgarian university rector faces dismissal over plagiarism"
"Years of Ethics Charges, but Star Cancer Researcher Gets a Pass"
"Institutional Research Misconduct Reports Need More Credibility"
"Tide of Lies"
"Researcher at the center of an epic fraud remains an enigma to those who exposed him"
"Quality of reports of investigations of research integrity by academic institutions"
"Research misconduct and the INTERGROWTH-21st study"
Research Misconduct (continued)
"Lawyer on the Norwegian system for investigating cheating in research: - No one understands how the law works"
When a researcher is suspected of cheating, the research institution must examine the case itself. It can go very wrong.
"Jurist om det norske systemet for å granske fusk i forskning: - Ingen skjønner hvordan loven fungerer"
Når en forsker mistenkes for juks, skal forskningsinstitusjonen selv granske saken. Det kan gå veldig galt.
"Scandal-weary Swedish government takes over research-fraud investigations"
"The Research Misconduct Board is one of the first national agencies tasked with investigating serious research misconduct."
Research Misconduct (continued)
"Stanford University's Research Policy HandbooK (RPH)"
"1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting"
Presents procedures for reporting and investigating allegations of research misconduct, and for the required notifications to federal agencies of such allegations and investigations.
"Lies, Damned Lies, and Medical Science"
"Dr. John Ioannidis Exposes the Bad Science of Colleagues - The Atlantic"
The Stanford Center for Clinical and Translational Research and Education, Stanford University
Meta-Research Innovation Center, Stanford University
Best Practices in Science (BPS Group), Stanford University
Research Misconduct (continued)
"To catch misconduct, journals are hiring research integrity czars"
"China introduces sweeping reforms to crack down on academic misconduct"
"Initiatives include a national list of cases, a blacklist of ‘poor quality’ journals and a government agency that is responsible for policing misconduct."
Research Misconduct (continued)
UK Research Integrity Office (UKRIO)
Research Misconduct (continued)
The Norwegian National Research Ethics Committees
Research Misconduct (continued)
Research Integrity in Norway (RINO) – a study of scientific misconduct and questionable research practices
Research Misconduct (continued)
"Research integrity: environment, experience, or ethos?"
The bottom-line, main message of a 2019 Norwegian study of students awarded their PhD degrees at the Faculty of Medicine at the University of Oslo in 2016 states: "Scientific misconduct appears to be an environmental problem as much as an issue of personal integrity."
"Unge forskere opplever uetisk press" (Young scientists are experiencing unethical pressure)
"They did a bad bad thing"
When it comes to research misconduct, burying one's head in the sand and pretending it doesn't exist is the worst possible plan.
- "With human nature as it is, the only surprising thing about scientific misconduct should be that it continues to surprise us. After all, scientists are human — with the same range of creativity, hopes, fears, anxieties and weaknesses as anyone else. Why should we be more surprised when scientists behave unethically than, say, those in business or politics? Surprised or not, we should acknowledge that scientific misconduct is happening, will always happen, and probably always has happened. With an increased awareness, however, we can all be more vigilant and perhaps better equipped to prevent it happening." (Source: "They did a bad bad thing" Editorial. Nature Chemistry volume 3, page 337 (2011) Published 20 April 2011 DOI https://doi.org/10.1038/nchem.1042)
Department of Health and Human Service, Office of Research Integrity
- Definition of Research Misconduct
Research misconduct means fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
(a) Fabrication is making up data or results and recording or reporting them.
(b) Falsification is manipulating research materials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented in the research record.
(c) Plagiarism is the appropriation of another person's ideas, processes, results, or words without giving appropriate credit.
(d) Research misconduct does not include honest error or differences of opinion.
(Source: U.S. Department of Health and Human Service, Office of Research Integrity. https://ori.hhs.gov/definition-misconduct) - "Avoiding Plagiarism, Self-plagiarism, and Other Questionable Writing Practices: A Guide to Ethical Writing"
"The purpose of this module is to help students, as well as professionals, identify and prevent questionable practices and to develop an awareness of ethical writing. This guide was written by Miguel Roig, PhD, from St. Johns University with funding from ORI."
Research Misconduct (continued)
- Why is research misconduct harmful?
Unfortunately, we have little knowledge of why researchers commit misconduct, but we do know that this reduces the confidence in published research results, and this makes the research less effective. Moreover, it is harmful for the research community's reputation, causing it to lose the trust of those who fund research. Research subjects who volunteer for projects may lose confidence, and public confidence in research may also suffer.
Misconduct may entail harmful or even fatal consequences, for example if the research in question is associated with treatment methods for patients, the safety of construction projects, environmental problems etc. (Gustafsson 2005). In addition, problems may arise if political decisions are made on the basis of dishonest or questionable research.
Finally, research misconduct will frequently be decidedly unfair to other colleagues: stealing a colleague's ideas, plagiarising research results produced by others etc. Another aspect related to colleagues is that funding of dishonest or questionable research comes at the cost of competing projects that abide by the requirements for good research practice. In particular, this is a problem in a situation of keen competition for strictly limited funding." (Source: "Fraud and plagiarism" Torkild Vinther. Last updated: 25. February 2016. The Norwegian National Research Ethics Committees)
"The European Code of Conduct for Research Integrity: Revised Edition"
("Europeiske retningslinjer for forskningsintegritet: REVIDERT VERSJON")
ALLEA - All European Academies
- "3. Violations of Research Integrity
It is of crucial importance that researchers master the knowledge, methodologies and ethical practices associated with their field. Failing to follow good research practices violates professional responsibilities. It damages the research processes, degrades relationships among researchers, undermines trust in and the credibility of research, wastes resources and may expose research subjects, users, society or the environment to unnecessary harm.
3.1 Research Misconduct and other Unacceptable Practices
Research misconduct is traditionally defined as fabrication, falsification, or plagiarism (the so-called FFP categorisation) in proposing, performing, or reviewing research, or in reporting research results:
• Fabrication is making up results and recording them as if they were real.
• Falsification is manipulating research materials, equipment or processes or changing, omitting or suppressing data or results without justification.
• Plagiarism is using other people’s work and ideas without giving proper credit to the original source, thus violating the rights of the original author(s) to their intellectual outputs.
These three forms of violation are considered particularly serious since they distort the research record. There are further violations of good research practice that damage the integrity of the research process or of researchers. In addition to direct violations of the good research practices set out in this Code of Conduct, examples of other unacceptable practices include, but are not confined to:
• Manipulating authorship or denigrating the role of other researchers in publications.
• Re-publishing substantive parts of one’s own earlier publications, including translations, without duly acknowledging or citing the original (‘self-plagiarism’).
• Citing selectively to enhance own findings or to please editors, reviewers or colleagues.
• Withholding research results.
• Allowing funders/sponsors to jeopardise independence in the research process or reporting of results so as to introduce or promulgate bias.
• Expanding unnecessarily the bibliography of a study.
• Accusing a researcher of misconduct or other violations in a malicious way.
• Misrepresenting research achievements.
• Exaggerating the importance and practical applicability of findings.
• Delaying or inappropriately hampering the work of other researchers.
• Misusing seniority to encourage violations of research integrity.
• Ignoring putative violations of research integrity by others or covering up inappropriate responses to misconduct or other violations by institutions.
• Establishing or supporting journals that undermine the quality control of research (‘predatory journals’).
Research Misconduct (continued)
"CHECKLIST: Policies and Procedures for Handling Research Misconduct Allegations"
"This checklist is used by the U.S. Department of Health and Human Services, Office of Research Integrity (ORI) and is intended only to provide general information regarding ORI’s review of institutional policies. " (Source: "CHECKLIST: Policies and Procedures for Handling Research Misconduct Allegations" Last Modified: 04/05/2017. U.S. Office of Research Integrity
"How to investigate allegations of research misconduct: A checklist"
"Do investigations into research misconduct allegations need better standards? The Association for the Promotion of Research Integrity (APRIN) in Japan, a group of volunteers who “commit themselves to the promotion of research of high integrity” and provide “e-learning material for research ethics education,” thinks so. Today, we present a guest post by Iekuni Ichikawa, who chaired an APRIN committee that recently came up with a new checklist for such investigations, about the effort. (Source: "How to investigate allegations of research misconduct: A checklist" Iekuni Ichikawa. Retraction Watch Posted on January 8, 2019; Also see: "Checklist for Investigating Allegations of Research Misconduct")
"Investigation of Allegations of Research Misconduct"
"Research misconduct is very narrowly defined as: (1) Fabrication of results, making up data; (2) Falsification of data, manipulating research materials, equipment or processes, or changing or omitting data or results such that the research is not accurately represented in the research record or; (3) Plagiarism, the appropriation of another person’s ideas, processes, results or words without giving credit.
Maintaining the highest ethical standards in the conduct of research remains the goal of the entire scientific community at the NIH. Our good reputation is essential for maintaining the trust of Congress, the American People, our Scientific Colleagues across the US and the International Community. It is the responsibility of each Investigator who participates in research at the NIH, no matter what their career stage, to maintain exemplary standard of intellectual honesty in formulating or conducting research and to report any suspected research misconduct to the NIH Agency Research Integrity Officer (ARIO)."
- NIH IRP Policies & Procedures for Research Misconduct Proceedings (PDF File)
- Guide to Handling of Research Misconduct Allegations (PDF File)
- Public Health Service Policies on Research Misconduct (PDF File)
(Source: "Investigation of Allegations of Research Misconduct" National Institutes of Health, Office of Intramural Research)
Research Misconduct (continued)
U.S. National Science Foundation (NSF)
Included below is the formal definition of research misconduct by the U.S. National Science Foundation (NSF), the largest funding organization of research and education in most fields of science and engineering.
(a) Research misconduct means fabrication, falsification, or plagiarism in proposing or performing research funded by NSF, reviewing research proposals submitted to NSF, or in reporting research results funded by NSF.
- Fabrication means making up data or results and recording or reporting them.
- Falsification means manipulating research materials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented in the research record.
- Plagiarism means the appropriation of another person’s ideas, processes, results or words without giving appropriate credit.
Research Misconduct (continued)
U.S. Federal Register, Part III, Department of Health and Human Services
CFR › Title 42 › Chapter I › Subchapter H › Part 93 › Subpart C › Section 93.310
"§ 93.310 Institutional investigation.
Institutions conducting research misconduct investigations must:
- (a) Time. Begin the investigation within 30 days after determining that an investigation is warranted.
- (b) Notice to ORI. Notify the ORI Director of the decision to begin an investigation on or before the date the investigation begins and provide an inquiry report that meets the requirements of § 93.307 and § 93.309.
- (c) Notice to the respondent. Notify the respondent in writing of the allegations within a reasonable amount of time after determining that an investigation is warranted, but before the investigation begins. The institution must give the respondent written notice of any new allegations of research misconduct within a reasonable amount of time of deciding to pursue allegations not addressed during the inquiry or in the initial notice of investigation.
- (d) Custody of the records. To the extent they have not already done so at the allegation or inquiry stages, take all reasonable and practical steps to obtain custody of all the research records and evidence needed to conduct the research misconduct proceeding, inventory the records and evidence, and sequester them in a secure manner, except that where the research records or evidence encompass scientific instruments shared by a number of users, custody may be limited to copies of the data or evidence on such instruments, so long as those copies are substantially equivalent to the evidentiary value of the instruments. Whenever possible, the institution must take custody of the records -
(1) Before or at the time the institution notifies the respondent; and
(2) Whenever additional items become known or relevant to the investigation. - (e) Documentation. Use diligent efforts to ensure that the investigation is thorough and sufficiently documented and includes examination of all research records and evidence relevant to reaching a decision on the merits of the allegations.
- (f) Ensuring a fair investigation. Take reasonable steps to ensure an impartial and unbiased investigation to the maximum extent practicable, including participation of persons with appropriate scientific expertise who do not have unresolved personal, professional, or financial conflicts of interest with those involved with the inquiry or investigation.
- (g) Interviews. Interview each respondent, complainant, and any other available person who has been reasonably identified as having information regarding any relevant aspects of the investigation, including witnesses identified by the respondent, and record or transcribe each interview, provide the recording or transcript to the interviewee for correction, and include the recording or transcript in the record of the investigation.
(h) Pursue leads. Pursue diligently all significant issues and leads discovered that are determined relevant to the investigation, including any evidence of additional instances of possible research misconduct, and continue the investigation to completion.
(Source: Cornell University, Cornell Law School, Legal Information Institute (LII) "42 CFR 93.310 - Institutional investigation." Also see: "42 CFR Part 93 - PUBLIC HEALTH SERVICE POLICIES ON RESEARCH MISCONDUCT" Also see: Part III, Department of Health and Human Services, 42 CFR Parts 50 and 93 Public Health Service Policies on Research Misconduct; Final Rule, Federal Register / Vol. 70, No. 94 / Tuesday, May 17, 2005 / Rules and Regulations) - Also see: Cornell Law School, Legal Information Institute
42 CFR Part 93 - PUBLIC HEALTH SERVICE POLICIES ON RESEARCH MISCONDUCT
https://www.law.cornell.edu/cfr/text/42/part-93
§ 93.103 Research misconduct.
Research misconduct means fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
- (a) Fabrication is making up data or results and recording or reporting them.
- (b) Falsification is manipulating researchmaterials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented in the research record.
- (c) Plagiarism is the appropriation of anotherperson's ideas, processes, results, or words without giving appropriate credit.
- (d) Research misconduct does not include honest error or differences of opinion.
(Source: Cornell University, Cornell Law School, Legal Information Institute (LII) "42 CFR 93.103 - Research misconduct.")
Research Misconduct (continued)
Stanford University
Research Policy Handbook
1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting
3. Definitions
"A. Research Misconduct
"Research misconduct" is defined as fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
- Fabrication means making up data or results, and recording or reporting them.
- Falsification means manipulating research materials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented in the research record.
- Plagiarism means the appropriation of another person's ideas, processes, results, or words without giving appropriate credit.
A finding of research misconduct requires that:
- there is a significant departure from accepted practices of the relevant research community
- the misconduct is committed intentionally, or knowingly, or recklessly
- the allegation is proven by a preponderance of the evidence. (Stanford University's disciplinary procedures may establish a different standard of proof for disciplinary actions
(Source: https://doresearch.stanford.edu/policies/research-policy-handbook/conduct-research/research-misconduct-policy-allegations-investigations-and-reporting#main-content)
"Understanding Stanford’s Code of Conduct"
- "The code is a shared statement of goals, rules and values designed to build trust, protect members of the university community and help people act with integrity. It applies to a broad group, ranging from faculty, staff, students and trustees to consultants, contractors and volunteers. Members of the university community can use the code as a guide to help identify and avoid problem areas, such as workplace, scientific and research misconduct; discrimination and harassment; conflicts of interest or commitment; privacy issues; and fraud, theft and embezzlement."
(...)
"Each of us is expected to act in accordance with the law and with university policies and procedures. As a member of the community, you are expected to raise concerns or report suspected violations of the code, even when these concerns or violations may not affect you directly. It is important to note that we consider this a service to the institution and have a strong non-retaliation policy for those who, in good faith, report or provide information about concerns or suspected violations.
The code supports our mission and vision by reinforcing the fact that we are each accountable for our own actions. As members of the university community, we are collectively accountable for upholding these standards of behavior, as well as for compliance with all applicable laws, regulations and policies that guide our work.
Stanford has high ideals. The code helps us live up to them and support the expectations that accompany them." (Source: "Understanding Stanford’s Code of Conduct" "Anne Sweeney Hoy, Stanford’s chief ethics and compliance officer, discusses the Code of Conduct, which is a tool kit for upholding Stanford’s high ethical standards." BY CHRIS PEACOCK. Stanford University News DECEMBER 11, 2018)
Research Misconduct (continued)
The International Association of Scientific, Technical and Medical Publishers (STM)
"The STM Report: An overview of scientific and scholarly publishing, Fifth edition, October 2018"
- "2.15 Publishing ethics
There has been a growing awareness of the need for higher (or at least more transparent) ethical standards in journal publishing to deal with issues such as conflict of interest, ghostwriting, guest authorship, citation rings, peer review rigging, authorship disputes, falsification and fabrication of data, scientific fraud, unethical experimentation and plagiarism. Much of the criticism has been addressed at the intersection of the biomedical journals and pharmaceutical industry but the issues are by no means unique to this sector." (Source: "The STM Report: An overview of scientific and scholarly publishing, Fifth edition, October 2018" Rob Johnson, Research Consulting; Anthony Watkinson, CIBER Research; Michael Mabe, The International Association of Scientific, Technical and Medical Publishers.)
"Scientific Misconduct and Medical Journals"
- "The Role and Responsibilities of Institutions
Institutions are expected to conduct an appropriate and thorough investigation of allegations of scientific misconduct. Some institutions are immediately responsive, acknowledging receipt of the letter from the journal describing the concerns, and quickly begin an investigation. In other cases, it may take time to identify the appropriate institutional individuals to contact, and even then, many months to receive a response. Some institutions appear well-equipped to conduct investigations, whereas other institutions appear to have little experience in such matters or fail to conduct adequate investigations 13; these institutions can take months to years to provide JAMA with an adequate response. In some cases involving questions of misconduct from outside of the United States, institutions have indicated that further investigation must wait until numerous legal issues are resolved, further delaying a response." (Source: "Scientific Misconduct and Medical Journals" Howard Bauchner, MD; Phil B. Fontanarosa, MD, MBA; Annette Flanagin, RN, MA; Joe Thornton, JD. Editorial, JAMA. Published online October 19, 2018. doi:10.1001/jama.2018.14350)
- "Duke has been hit by multiple high-profile misconduct cases in recent years. One such case involved Anil Potti, a once-rising star in cancer research who fabricated data, leading to 12 retractions, multiple lawsuits, and reprimands from the medical board. The university is also involved in an ongoing lawsuit filed by a whistleblower, which alleges that a pulmonary scientist, her supervisor, and the university included fraudulent data in federal documents associated with more dozens of grants worth hundreds of millions of dollars." (Source: "Duke’s mishandling of misconduct prompts new U.S. government grant oversight" By Alison McCook, Retraction Watch in Science Mar. 23, 2018 , 4:15 PM) [Note: See: "Case Summary: Potti, Anil" and "Notice is hereby given that the Office of Research Integrity (ORI) has taken final action in the following case: Anil Potti, M.D., Duke University School of Medicine"]
"Duke University to settle case alleging researchers used fraudulent data to win millions in grants"
- "According to court documents filed last week in the Middle District of North Carolina in Greensboro, former Duke biologist Joseph Thomas, who sued the university in 2015 under a federal law that allows whistleblowers to receive as much as 30% of any payout, is waiting for the U.S. Department of Justice to approve the settlement. Thomas brought his case under the federal False Claims Act (FCA), which could force Duke to return to the government up to three times the amount of any ill-gotten funds." (Source: "Duke University to settle case alleging researchers used fraudulent data to win millions in grants" By Ivan Oransky, Retraction Watch Nov. 19, 2018, 12:10 PM. Science. Posted in: Scientific Community. doi:10.1126/science.aaw1185)
"It’s time to end the code of silence at universities"
- "Although two Federal agencies — the U.S. Office of Research Integrity and the National Science Foundation’s Office of the Inspector General — do have oversight of university investigations into scientific misconduct, the public has no way of knowing whether such investigations were carried out properly. As we and a colleague wrote in the Journal of the American Medical Association earlier this year, when universities investigate their own — which by Federal statute, they are obligated to do — they have serious conflicts of interest, and the resulting investigations are often deeply flawed." (Source: "It’s time to end the code of silence at universities" Ivan Oransky. Retraction Watch November 6, 2018)
"The association between quality measures of medical university press releases and their corresponding news stories—Important information missing"
- "Conclusions
This large study of medical university press releases and corresponding news stories showed that important measures of a scientific study such as funding and study limitations were omitted to a very large extent. The lay public and health personnel as well as policy makers, politicians and other decision makers may be misled by incomplete and partly inaccurate representations of scientific studies which could negatively affect important health-related behaviours and decisions." (Source: "The association between quality measures of medical university press releases and their corresponding news stories—Important information missing" Maike Winters , Anna Larsson, Jan Kowalski, Carl Johan Sundberg. PLOS|ONE https://doi.org/10.1371/journal.pone.0217295 June 12, 2019)
"Bulgarian university rector faces dismissal over plagiarism"
- "Bulgaria’s Education Minister will sack the rector of the Technical University of Varna, Rossen Vassilev, after an ethics commission found evidence of extensive plagiarism in his doctoral dissertation.
It is a first for Bulgaria, after amendments passed earlier this year created the legal grounds for an academic ethics commission at the Education Ministry. The amendments were, in part, prompted by the highly-publicised accusations against Vassilev, which first surfaced last year and made by some of colleagues at the university.
At the time, the law’s provisions only allowed for a court challenge from the plagiarised authors.
Under the amended law, Vassilev’s doctoral dissertation and scientific publications he presented to be granted the professor title were reviewed by three arbiters. Their findings – namely that Vassilev plagiarised more than 90 per cent of his dissertation, including spelling and grammar mistakes in the source material – were the grounds for the ethics commission to rule that Vassilev was guilty of plagiarism.
Bulgaria’s Education Minister Krassimir Vulchev said that Vassilev will be stripped of this doctoral degree, would lose his professor title and the position as rector of the university. An interim rector would be appointed by the minister next week, who would terminate Vassilev’s employment contract.
But the law does not prevent Vassilev from retaining his position as a lecturer at the university – docent, in Bulgarian academia parlance – and could even be re-elected rector by the university’s general assembly, one media report claimed.
Vassilev previously denied the accusations against him and the Technical University of Varna published a lengthy rebuttal of the ethics committee’s decision, questioning the motivation of Vassilev’s accusers and the credentials of the arbiters selected to review his dissertation.
It also warned that the ethics commission was being used for a “witch hunt” and said that any disputes about plagiarism should be settled in court." (Source: "Written by The Sofia Globe staff on November 9, 2018 in Bulgaria - Comments Off on Bulgarian university rector faces dismissal over plagiarism")
"Years of Ethics Charges, but Star Cancer Researcher Gets a Pass"
- "'A Tremendous Conflict of Interest'
Within the realm of biomedical science, it falls to the Office of Research Integrity to issue formal findings of scientific misconduct, which can lead to suspension of federal financing and effectively end a research career. The office labors under an awkward constraint: It does not carry out its own investigations, but relies on accused researchers’ own institutions to forward their findings.
With their own reputations on the line, institutions “have a tremendous conflict of interest,” said Dr. Richard Smith, former editor of The British Medical Journal and a founding member of the Committee on Publication Ethics in Britain. “There’s a terrible temptation to bury it all,” he added.
There are also dollars at stake. Of the $29.1 million Dr. Croce has received in federal funding as a principal investigator while at Ohio State, university records show, $8.7 million has gone directly to the university in overhead payments, a fairly standard cut for research institutions." (Source: "Years of Ethics Charges, but Star Cancer Researcher Gets a Pass" By James Glanz and Agustin Armendariz. The New York Times March 8, 2017. "A version of this article appears in print on March 9, 2017, on Page A1 of the New York edition with the headline: Years of Questions, but Researcher Gets a Pass.") [Also see: "Judge dismisses most of Carlo Croce’s libel case against the New York Times" in Retraction Watch. Ivan Oransky. November 8, 2018]
"Institutional Research Misconduct Reports Need More Credibility"
- "As a result, like most elements of research misconduct, little is known about institutions’ responses to potential misconduct by their own members. The community that relies on the integrity of university research does not have access to information about how often such claims arise, or how they are resolved. Nonetheless, there are some indications that many internal reviews are deficient.
(...)
The Office of Inspector General (OIG) of the National Science Foundation (NSF) has found that the reports of some institutions do not meet reasonable standards. For instance, some reports from universities do not ask relevant research questions, or they fail to appropriately expand the investigation beyond a particular allegation; other reports focus on finding fault with an individual when many were involved in the research; and for some other reports, committee members have lacked relevant expertise (email from Alan Boehm, MFS; James T. Kroll, PhD; and Aaron S. Manka, PhD; December 2017). These are not idiosyncratic or 1-time problems. A partial list of shortcomings that the OIG staff has compiled and shared at conferences includes the following:- Investigative reports that lack supporting evidence and fail to address the elements of a research misconduct finding, particularly intent;
- Individuals who are the subjects of the investigation blaming the student or postdoctoral researchers, but the investigative committee never interviewing these individuals;
- Accepting, without question, excuses by the subjects of the investigation; and
- Relying only on information in allegations, not checking for patterns or other misconduct."
- "The group developed a proposed checklist for research integrity investigation (eAppendix in the Supplement). The checklist, or its improved successors, is designed to address whether an investigation follows reasonable standards and if the subsequent report is appropriate and complete. For example, it addresses the following issues:
- Whether a specific institutional investigation plan or report is generated that identifies appropriate questions to pursue and proposes a meaningful approach to securing the answers;
- Whether the correct individuals are interviewed;
- Whether the relevant data are secured and reviewed by appropriate experts;
- Whether the report provides factual basis and data; and
- Whether the report supports its conclusions." (Source: "Institutional Research Misconduct Reports Need More Credibility" C. K. Gunsalus, JD; Adam R. Marcus, MA; Ivan Oransky, MD. JAMA. 2018;319(13):1315-1316. doi:10.1001/jama.2018.0358)
"Tide of Lies"
"Researcher at the center of an epic fraud remains an enigma to those who exposed him"
- "Summary
Japanese bone researcher Yoshihiro Sato fabricated dozens of clinical trials before a group of researchers from the United Kingdom and New Zealand exposed the fraud. The impact of his fabricated reports—many of them on how to reduce the risk of bone fractures—rippled far and wide. Meta-analyses that included his trials came to the wrong conclusion; professional societies based medical guidelines on his papers. Following up on the faked studies, researchers carried out large new trials that had enrolled thousands of real patients. Exposing Sato's lies, correcting the literature, and tracing the effects of the fake studies has been a bruising struggle for the whistleblowers. But the sprawling fraud case has also had a massive impact on those who worked with Sato and on Sato himself."
"Sato's fraud was one of the biggest in scientific history. The impact of his fabricated reports—many of them on how to reduce the risk of bone fractures—rippled far and wide. Meta-analyses that included his trials came to the wrong conclusion; professional societies based medical guidelines on his papers. To follow up on studies they did not know were faked, researchers carried out new trials that enrolled thousands of real patients. Exposing Sato's lies and correcting the literature had been a bruising struggle for Avenell and her colleagues.
Yet they could not understand why Sato faked so many studies, or how he got away with it for so long. They puzzled over the role of his co-authors, some of whom had their names on dozens of his papers. ("Do we honestly believe they knew nothing at all about what was going on?" Avenell asked.) They wondered whether other doctors at his hospital read Sato's work—and whether the Japanese scientific community ever questioned how he managed to publish more than 200 papers, many of them ambitious studies that would have taken most researchers years to complete." (Source: "Tide of Lies" "Researcher at the center of an epic fraud remains an enigma to those who exposed him " Kai Kupferschmidt. Science 17 Aug 2018: Vol. 361, Issue 6403, pp. 636-641. doi:10.1126/science.aav1079. Also see: http://science.sciencemag.org/content/361/6403/636)
"Quality of reports of investigations of research integrity by academic institutions"
- "Background
Academic institutions play important roles in protecting and preserving research integrity. Concerns have been expressed about the objectivity, adequacy and transparency of institutional investigations of potentially compromised research integrity. We assessed the reports provided to us of investigations by three academic institutions of a large body of overlapping research with potentially compromised integrity. - Methods
In 2017, we raised concerns with four academic institutions about the integrity of > 200 publications co-authored by an overlapping set of researchers. Each institution initiated an investigation. By November 2018, three had reported to us the results of their investigations, but only one report was publicly available. Two investigators independently assessed each available report using a published 26-item checklist designed to determine the quality and adequacy of institutional investigations of research integrity. Each assessor recorded additional comments ad hoc. - Results
Concerns raised with the institutions were overlapping, wide-ranging and included those which were both general and publication-specific. The number of potentially affected publications at individual institutions ranged from 34 to 200. The duration of investigation by the three institutions which provided reports was 8–17 months. These investigations covered 14%, 15% and 77%, respectively, of potentially affected publications. Between-assessor agreement using the quality checklist was 0.68, 0.72 and 0.65 for each report. Only 4/78 individual checklist items were addressed adequately: a further 14 could not be assessed. Each report was graded inadequate overall. Reports failed to address publication-specific concerns and focussed more strongly on determining research misconduct than evaluating the integrity of publications. - Conclusions
Our analyses identify important deficiencies in the quality and reporting of institutional investigation of concerns about the integrity of a large body of research reported by an overlapping set of researchers. They reinforce disquiet about the ability of institutions to rigorously and objectively oversee integrity of research conducted by their own employees." (Source: "Quality of reports of investigations of research integrity by academic institutions" Andrew Grey, Mark Bolland, Greg Gamble and Alison Avenell. Research Integrity and Peer Review20194:3. https://doi.org/10.1186/s41073-019-0062-x Received: 9 December 2018, Accepted: 5 February 2019, Published: 19 February 2019) [See: New study finds “important deficiencies” in university reports of misconduct" Ivan Oransky. Retraction Watch February 25, 2019]
"Research misconduct and the INTERGROWTH-21st study"
- "We respectfully take issue with the Comment published in February, 2017, by members of The Lancet's editorial staff stating that WHO's judgment of misconduct regarding the Oxford INTERGROWTH-21st study was unproven. 1,2,3. We are saddened by the fact that they base their conclusion on limited and biased material and simply dismiss the case as rivalry. As members of the WHO Fetal Growth research group and a former WHO external expert not associated with that trial but with evidence that WHO initially refused to hear, we wish to note the following.
The Lancet editors presented the case as if the allegation of misconduct consisted merely of plagiarism or duplication of intellectual content, and therefore, did not mention the more decisive issues. These issues are more serious than those mentioned in the Comment, and stimulate us to outline them here.
José Villar and Stephen Kennedy were members of our external advisory team, which was involved in preparing the WHO Fetal Growth Study. Forms were signed indicating no competing interests and both had also received a stipend to develop the protocol for the WHO Fetal Growth study. Months later, after delaying submitting their contracted work, they revealed that they had received a grant from the Gates Foundation to do the study at Oxford University. They apologised and offered to return the WHO stipend. We believe this application not only included intellectual content gleaned from their WHO committee but also had a significant effect on the WHO study. Unfortunately, WHO then clearly lacked the guidelines or instruments to investigate our concerns about scientific misconduct. Instead, the case was discussed only at a higher administrative level between WHO and Oxford University, without consulting our group, the very investigators involved in the WHO study.
When we learned that WHO and Oxford University were about to settle the case without having addressed our major concerns about misconduct—including the details of the competing interests statement, not fulfilling the commission covered by the stipend, and delaying the WHO study—merely on the basis of the question of plagiarism, our research team immediately reacted.
The entire WHO Fetal Growth Study research team demanded an independent investigation including all issues and parties. WHO agreed, but Oxford University disagreed. We still do not understand why an institution such as Oxford University repeatedly should decline an independent review of concerns about scientific misconduct, unless they had something to hide. We do not feel that an institution for whom a grant of £29 million from the Bill & Melinda Gates Foundation was at stake can be regarded as unbiased in this case.
In our view, Oxford University's claim of complete investigation is spurious. They have never sought data from ourselves, nor, equally importantly, from one of their own former faculty members who had been on the WHO Fetal Growth Study advisory committee.
Transparency does not seem to have been the involved institutions' priority. We never received access to the full report of Oxford University's assessment, the external risk assessment commissioned by WHO, the questions WHO requested another four external experts to address or the content of their report, or the letter that WHO sent to the General Medical Council. Interestingly, The Lancet editors received some of these documents.
We also wish to clarify that the Committee on Publication Ethics (COPE) did not look into the merits of the charges, but only into matters of due process at the journals affected.
The handling of this case appears to us as an attempt to minimise the problem and to try to make it disappear silently. We have patiently pressed for an orderly independent review of all sides of these allegations instead of the present public show, but are increasingly concerned that large powerful organisations seem to have a different attitude towards ethics and how to handle related challenges.
This is far from a complete account of this saga, but we had hoped that transparency and fairness should prevail and we were prepared to accept an independent assessment of all parts of the allegation.
As it stands now, it looks to us like taking the intellectual content from the WHO fetal growth study to another project and, receiving money for developing the study and then not doing it, while having declared no conflict of interest, is approved scientific conduct.
We declare no competing interests apart from the issues mentioned in this letter, and that LP is external advisor to WHO, chair of the steering committee of the WHO Fetal Growth study, and he receives research support from GE Medical Systems; TK is external advisor to WHO and member of the Steering committee of the WHO Fetal Growth study; and ML has acted as external advisor to WHO in two former studies." (Source: "Research misconduct and the INTERGROWTH-21st study" Lawrence D Platt, Torvid Kiserud, Marshall Lindheimer. The Lancet, CORRESPONDENCE, VOLUME 390, ISSUE 10099, P1025-1026, SEPTEMBER 09, 2017. Published: August 22, 2017. DOI: https://doi.org/10.1016/S0140-6736(17)31365-X. View/Download pdf here.)
Research Misconduct (continued)
"Lawyer on the Norwegian system for investigating cheating in research: - No one understands how the law works"
When a researcher is suspected of cheating, the research institution must examine the case itself. It can go very wrong.
"Jurist om det norske systemet for å granske fusk i forskning: - Ingen skjønner hvordan loven fungerer"
Når en forsker mistenkes for juks, skal forskningsinstitusjonen selv granske saken. Det kan gå veldig galt.
- "Special law in Norway
If a researcher cheats in his research, there is today a principle in Norway that the case should be examined and treated by the same institution that the person in question works or has worked.
This was decided in a new law on research ethics that came in 2017.
The advantage is that the process can get started quickly. One of the major drawbacks, on the other hand, is that those who control the survey often know the accused and have an interest in the institution's name and reputation.
This is probably the reason why Denmark's new law on research ethics is completely different from Norway's
There is the opposite principle: Colleagues should not scrutinize colleagues. Instead, an external committee will investigate the most serious cases of research fraud.
Sweden is about to get a new law on research ethics. They seem to go the same way as the Danes. They propose, after a thorough assessment of the various models used in other countries, that a suspicion of misconduct be reported to a national "Misconduct Board"."
"Spesiell lov i Norge
Om en forsker jukser i forskningen sin, er det i dag et prinsipp i Norge at saken skal undersøkes og behandles ved den samme institusjon som vedkommende jobber eller har jobbet.
Dette ble bestemt i en ny forskningsetikklov som kom i 2017.
Fordelen er at prosessen raskt kan komme i gang. En av de store ulempene er derimot at de som styrer granskningen gjerne kjenner den anklagede, og har en interesse i institusjonens navn og rykte.
Det er trolig bakgrunnen for at Danmarks nye forskningsetikklov er helt annerledes enn Norges.
Der er prinsippet motsatt: Kolleger ikke skal granske kolleger. I stedet skal et eksternt utvalg undersøke de alvorligste sakene av forskningsjuks.
Sverige er i ferd med å få en ny forskningsetikklov. De ser ut til å gå samme vei som danskene. De foreslår, etter en grundig vurdering av de ulike modellene som brukes i andre land, at en mistanke om uredelighet skal meldes til en nasjonal «Oredelighetsnemd»." (Source: "Lawyer on the Norwegian system for investigating cheating in research: - No one understands how the law works" "Jurist om det norske systemet for å granske fusk i forskning: - Ingen skjønner hvordan loven fungerer" Siw Ellen Jakobsen & Ingrid Spilde. Forskning.no. 09 May 2019)
"Scandal-weary Swedish government takes over research-fraud investigations"
"The Research Misconduct Board is one of the first national agencies tasked with investigating serious research misconduct."
- "Bruised by a string of high-profile scientific-misconduct cases, Sweden has laid the legislative groundwork for a government agency that will handle all allegations of serious research misconduct. The country follows in the footsteps of neighbouring Denmark, which created the world’s first such agency in 2017." (Source: "Scandal-weary Swedish government takes over research-fraud investigations" Holly Else. Nature 571, 158 (2019) doi: 10.1038/d41586-018-05493-3)
- "The way in which Swedish research institutes handle allegations of research misconduct has come under fire in recent years — thanks in part to the case of trachea surgeon Paolo Macchiarini. Macchiarini had been accused of misconduct relating to trials of an experimental trachea-transplant method, in which some patients died. On three occasions in 2015, the prestigious Karolinska Institute in Stockholm cleared him, but independent investigations commissioned by the Karolinska later found that he had committed misconduct. A 2016 independent commission concluded that the institute’s procedures were flawed." (Source: Ibid.)
- "The agency will be created through a law that passed parliament on 18 June and is expected to come into force in January 2020. The law defines research misconduct as fabrication, falsification or plagiarism. All cases of alleged serious research misconduct at publicly funded research institutes will be referred to the board for investigation. Findings will be made public and will be legally binding for universities, which will be allowed to decide the consequences for the researcher." (Source: Ibid.)
- "Other countries are looking at how they deal with research misconduct. In July 2017, Denmark established a national system similar to Sweden’s. It has so far investigated nine cases." (Source: Ibid.)
Research Misconduct (continued)
"Stanford University's Research Policy HandbooK (RPH)"
- Policies
"1. Conduct of Research
1.1 Principles Concerning Research
1.2 Rights and Responsibilities in the Conduct of Research
1.3 Academic Freedom
1.4 Openness in Research
1.5 On Academic Authorship
1.6 Multi-Authored Research Papers
1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting
1.8 Nondiscrimination in Research Agreements
1.9 Retention of and Access to Research Data"
(Source: https://doresearch.stanford.edu/policies/research-policy-handbook)
"1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting"
Presents procedures for reporting and investigating allegations of research misconduct, and for the required notifications to federal agencies of such allegations and investigations.
- Jump To:
1. Introduction
2. Applicability
3. Definitions
4. Federal Funding Agency Requirements
5. Individual Reporting Responsibility
6. Procedure for School Dean's Review
7. Internal Coordination/Reports to the Dean of Research
8. Notification to External Agencies
9. Determination of Discipline
10. Cautions and Assistance"
(Source: Stanford University: https://doresearch.stanford.edu/policies/research-policy-handbook/conduct-research/research-misconduct-policy-allegations-investigations-and-reporting#main-content)
"Lies, Damned Lies, and Medical Science"
"Dr. John Ioannidis Exposes the Bad Science of Colleagues - The Atlantic"
- "Much of what medical researchers conclude in their studies is misleading, exaggerated, or flat-out wrong. So why are doctors—to a striking extent—still drawing upon misinformation in their everyday practice? Dr. John Ioannidis has spent his career challenging his peers by exposing their bad science.
(...)
But beyond the headlines, Ioannidis was shocked at the range and reach of the reversals he was seeing in everyday medical research. “Randomized controlled trials,” which compare how one group responds to a treatment against how an identical group fares without the treatment, had long been considered nearly unshakable evidence, but they, too, ended up being wrong some of the time. “I realized even our gold-standard research had a lot of problems,” he says. Baffled, he started looking for the specific ways in which studies were going wrong. And before long he discovered that the range of errors being committed was astonishing: from what questions researchers posed, to how they set up the studies, to which patients they recruited for the studies, to which measurements they took, to how they analyzed the data, to how they presented their results, to how particular studies came to be published in medical journals.
This array suggested a bigger, underlying dysfunction, and Ioannidis thought he knew what it was. “The studies were biased,” he says. “Sometimes they were overtly biased. Sometimes it was difficult to see the bias, but it was there.” Researchers headed into their studies wanting certain results—and, lo and behold, they were getting them. We think of the scientific process as being objective, rigorous, and even ruthless in separating out what is true from what we merely wish to be true, but in fact it’s easy to manipulate results, even unintentionally or unconsciously. “At every step in the process, there is room to distort results, a way to make a stronger claim or to select what is going to be concluded,” says Ioannidis. “There is an intellectual conflict of interest that pressures researchers to find whatever it is that is most likely to get them funded."
(...)
THOUGH SCIENTISTS AND science journalists are constantly talking up the value of the peer-review process, researchers admit among themselves that biased, erroneous, and even blatantly fraudulent studies easily slip through it. Nature, the grande dame of science journals, stated in a 2006 editorial, “Scientists understand that peer review per se provides only a minimal assurance of quality, and that the public conception of peer review as a stamp of authentication is far from the truth.” What’s more, the peer-review process often pressures researchers to shy away from striking out in genuinely new directions, and instead to build on the findings of their colleagues (that is, their potential reviewers) in ways that only seem like breakthroughs—as with the exciting-sounding gene linkages (autism genes identified!) and nutritional findings (olive oil lowers blood pressure!) that are really just dubious and conflicting variations on a theme.
(...)
Perhaps only a minority of researchers were succumbing to this bias, but their distorted findings were having an outsize effect on published research. To get funding and tenured positions, and often merely to stay afloat, researchers have to get their work published in well-regarded journals, where rejection rates can climb above 90 percent. Not surprisingly, the studies that tend to make the grade are those with eye-catching findings. But while coming up with eye-catching theories is relatively easy, getting reality to bear them out is another matter. The great majority collapse under the weight of contradictory data when studied rigorously. Imagine, though, that five different research teams test an interesting theory that’s making the rounds, and four of the groups correctly prove the idea false, while the one less cautious group incorrectly “proves” it true through some combination of error, fluke, and clever selection of data. Guess whose findings your doctor ends up reading about in the journal, and you end up hearing about on the evening news? Researchers can sometimes win attention by refuting a prominent finding, which can help to at least raise doubts about results, but in general it is far more rewarding to add a new insight or exciting-sounding twist to existing research than to retest its basic premises—after all, simply re-proving someone else’s results is unlikely to get you published, and attempting to undermine the work of respected colleagues can have ugly professional repercussions." (Source: "Lies, Damned Lies, and Medical Science" David H. Freedman. The Atlantic November 2010 Issue)
The Stanford Center for Clinical and Translational Research and Education, Stanford University
- "The mission of Spectrum, Stanford’s Clinical and Translational Science Award (CTSA)-supported research hub, is to transform clinical and translational research and education at Stanford to make it more effective at discovering and implementing data-driven strategies to serve the health needs of individuals and the population. Spectrum’s programs extend from the earliest stages of the translational pipeline to the “final mile” of implementation science at the patient and population levels. Spectrum leverages local achievements, institutional strengths and resources, and advancements in the focus of the CTSA consortium to nurture an innovative and collaborative workforce that can transfer health discoveries from the University to patients and the community."
Meta-Research Innovation Center, Stanford University
- Goals
- Build the meta-research field and catalyze solutions-focused research to develop best scientific practices
- Provide leaders with the skills and knowledge needed to support the development and implementation of high-quality research
- Transform research practices to strengthen the evidence base for informed decision-making
Best Practices in Science (BPS Group), Stanford University
Research Misconduct (continued)
"To catch misconduct, journals are hiring research integrity czars"
- "Scientific journals’ creation of dedicated positions for rooting out misconduct before publication comes amid growing awareness of such issues, and stems from a recognition that spot-checking and other ad hoc arrangements were insufficient" (Source: "To catch misconduct, journals are hiring research integrity czars" By IVAN ORANSKY and ADAM MARCUS. STAT NOVEMBER 21, 2018)
"China introduces sweeping reforms to crack down on academic misconduct"
"Initiatives include a national list of cases, a blacklist of ‘poor quality’ journals and a government agency that is responsible for policing misconduct."
- "China is getting tough on scientific misconduct. The country’s most powerful bodies, the Chinese Communist Party and the State Council, introduced a raft of reforms on 30 May aimed at improving integrity across the research spectrum, from funding and job applications to peer-review and publications.
Under the new policy, the Ministry of Science and Technology (MOST) will be responsible for managing investigations and ruling on cases of scientific misconduct, a role previously performed by individual institutions. And for the first time, misconduct cases will be logged in a national database that is currently being designed by MOST.
Inclusion in the list could disqualify researchers from future funding or research positions, and might also affect their ability to get jobs outside academia. The Chinese Academy of Social Sciences will oversee the same process for social scientists." (Source: "China introduces sweeping reforms to crack down on academic misconduct" David Cyranoski. Nature VOL 558. 08 June 2018. p. 171. View/Download pdf here.)
Research Misconduct (continued)
UK Research Integrity Office (UKRIO)
- Included below is the formal definition of research misconduct by the UK Research Integrity Office (UKRIO).
"3.16 Misconduct in research
3.16.1 Organisations should define what they consider to be misconduct in research and make it known to researchers
UKRIO defines misconduct in research as including, but not limited to:
a) Fabrication;
b) Falsification;
c) Misrepresentation of data and/or interests and/or involvement;
d) Plagiarism; and
e) Failures to follow accepted procedures or to exercise due care in carrying out responsibilities for:
i) avoiding unreasonable risk or harm to:
- humans;
- animals used in research; and
- the environment; and
ii) the proper handling of privileged or private information on individuals collected during the research."
(Source: UK Research Integrity Office, 3.16 Misconduct in research)
Research Misconduct (continued)
The Norwegian National Research Ethics Committees
- "The National Committee for Research Ethics in Science and Technology (NENT) is part of the Norwegian National Research Ethics Committees, an independent administrative agency under the Ministry of Education and Research. NENT is an impartial advisory body providing guidance and advice on research ethics, and the guidelines are important tools for promoting good scientific practice in the Norwegian research system." (Source: Back cover of: "Guidelines for research ethics in science and technology" Issued by The Norwegian National Committee for Research Ethics in Science and Technology (2016), p. 10. Text: The Norwegian National Committees for Research Ethics, Last updated: Tuesday, June 28, 2016. ISBN 978-82-7682-075-1)
- Fraud and plagiarism
"It may be tempting to dress up in borrowed plumage (plagiarise/refrain from referring to one’s sources), cook data (falsify data) or simply invent (fabricate) data, and disregard the fact that this violates fundamental and internationally accepted rules for good research practice." (Source: "Fraud and plagiarism" Text: Torkild Vinther, Last updated: 25. February 2016. The Norwegian National Research Ethics Committees) - "Scientific integrity
Researchers are responsible for respecting the research results of others and for exercising good scientific practice. Researchers must not conceal, misrepresent or falsify anything, whether in the planning, execution or reporting of the research. Plagiarism involves presenting the ideas or research of others as one’s own.
The individual researcher has an independent responsibility not to accept departures from good scientific practice, on his or her own account or that of others. 2 Researchers who discover or are made aware of errors in their research, must admit the error, correct it, and ensure that the consequences of the error are minimal."
(Source: "Guidelines for research ethics in science and technology" Issued by The Norwegian National Committee for Research Ethics in Science and Technology (2016), p. 10. Text: The Norwegian National Committees for Research Ethics, Last updated: Tuesday, June 28, 2016. ISBN 978-82-7682-075-1) - "Good citation practice
It is in the nature of research to build on research by others. Researchers who take advantage of the ideas and research by others, both published and unpublished, must acknowledge this accurately, so that it is clear what the researcher’s own contribution is. Researchers must give a balanced and correct presentation of the research of others. Citations make research traceable and verifiable."
[2 The Act on ethics and integrity in research (the Research Ethics Act).] (Source: ibid.) - "5 Researchers must respect the contributions of other researchers and observe standards of authorship and cooperation.
Researchers must observe good publication practice. They must clarify individual responsibilities in group work as well as the rules for co-authorship. Honorary authorship is unacceptable. When several authors contribute, each authorship must be justified. Justified authorship is defined by four criteria, in accordance with the criteria drawn up by the International Committee of Medical Journal Editors (ICMJE) 3 :
a) Researchers must have made a substantial contribution to the conception and design or the data acquisition or the data analysis and interpretation; and
b) researchers must have contributed to drafting the manuscript or critical revision of the intellectual content of the publication; and
c) researchers must have approved the final version before publication; and
d) researchers must be able to accept responsibility for and be accountable for the work as a whole (albeit not necessarily all technical details) unless otherwise specified.
All authors in a multidisciplinary publication must be able to account for the part or parts for which they have been responsible in the research work, and which part or parts are the responsibility of other contributors.
All those who meet criterion a) must be able to meet b) and c). Contributors who do not fulfil all the criteria must be acknowledged.
Research Misconduct (continued)
Research Integrity in Norway (RINO) – a study of scientific misconduct and questionable research practices
- Project to investigate research integrity among all researchers in Norway
"The project, called RINO – Research Integrity in Norway, is a collaboration between UiB, The Norwegian National Research Ethics Committees (FEK) and The Western Norway University of Applied Sciences (HVL). Helene Ingierd, one of FEKs representatives to the RINO-project, says it can provide important knowledge."
"The National Research Ethics Committees (FEK) is an advisory body in the field of research ethics, and we are also supposed to spread knowledge. In order to exert this role in the best possible way, we need a knowledge base", said Ingierd." (Source: The Norwegian National Research Ethics Committee website: Project to investigate research integrity among all researchers in Norway. Text: Ingrid S. Torp, Last updated: 12. February 2018. Also see RINO Project PDF:Research Integrity in Norway (RINO) – a study of scientific misconduct and questionable research practices)
- The RINO Project is featured on the website of the European Network of Research Integrity Offices (ENRIO). Norway is a founding member of ENRIO. Torkild Vinther, Director of The National Commission for the Investigation of Research Misconduct, is Vice-Chair of ENRIO. Moreover, Torkild Vinther is a member of the Reference Group for the RINO Project. (Source: ENRIO website: http://www.enrio.eu/news-activities/research-integrity-norway-rino/, RINO Project website: https://www.uib.no/en/rino and RINO Project First Interim Report PDF)
- Torkild Vinther authored a book chapter titled "SKJØNNSUTØVELSE I STADFESTING AV PLAGIAT" ("EXERCISE DISCRETION IN CONFIRMATION OF PLAGIARISM") in: Etisk skjønn i forskning (Ethical discretion in research). Included below is an excerpt form this article in which Torkild Vinther makes the important point that along with text plagiarism, failure to credit the source of one's ideas is also plagiarism.
- "Although the main focus is text plagiarism, it is still necessary to mention explicitly where you have acquired your ideas (models, concepts, hypotheses, explanations, metaphors, operationalizations, conclusions, etc.). Cosmetic or minor changes do not remove the requirement to credit the source. If you do not, it will appear that the idea is one's own."
"Selv om hovedfokus er tekstplagiat, skal det likevel nevnes at man eksplisitt må vise til hvor man har hentet sine ideer (modeller, begreper, hypoteser, forklaringer, metaforer, operasjonaliseringer, konklusjoner osv.). Kosmetiske eller mindre endringer fjerner ikke kravet om å kreditere kilden. Gjør man ikke det, vil det fremstå som om ideen er ens egen." (Source: "SKJØNNSUTØVELSE I STADFESTING AV PLAGIAT" in Etisk skjønn i forskning edited by Hallvard J. Fossheim og Helene Ingierd 2015 (Redaksjonelt arbeid, utvalg og introduksjon (Editorial work, selection and introduction)) p. 87-98) [Note: About the editors: Hallvard J. Fossheim is Professor of Philosophy, University of Bergen and the (2010-2014) secretariat leader of The National Research Ethical Committee for Social Sciences and Humanities (NESH) and the National Committee for the Evaluation of Human Resource Research (Skelettutvalget); and, Helene Ingierd is Director, The National Committee for Research Ethics in Science and Technology (NESH)]
- "Although the main focus is text plagiarism, it is still necessary to mention explicitly where you have acquired your ideas (models, concepts, hypotheses, explanations, metaphors, operationalizations, conclusions, etc.). Cosmetic or minor changes do not remove the requirement to credit the source. If you do not, it will appear that the idea is one's own."
- The RINO Project’s first interim report (June 2018) showed the percentages of the RINO survey respondents who know colleagues who have participated in questionable or unacceptable practices of Fabrication, Falsification & Plagiarism over the last 3 years to be 2.2%, 2.2% & 13.8%, respectively (Table 7, p. 16/English; 13/Norwegian). It would appear Plagiarism is more than 6 times more prevalent than Fabrication and Falsification.
- "The RINO survey shows that, of the three mentioned practices, knowledge of colleagues’ plagiarism is far more widespread than knowledge of colleagues fabricating or falsifying data."
"Almost 14%, or around 1 in 7 respondents, state that they are aware of colleagues plagiarising others’ work in the last three years. The corresponding figure for fabricating or falsifying data is just 2%, or 1 in 50." (Source: English: "Research integrity in Norway – results from a nationwide survey on research ethics" Johs. Hjellbrekke, Laura Drivdal, Helene Ingierd, Ole Bjørn Rekdal, Heidi Skramstad, Ingrid S. Torp og Matthias Kaiser. 2018. p. 16. ISBN: 978-82-7682-082-9) [Note: RINO Project survey questionnaire; RINO website: RESEARCH INTEGRITY IN NORWAY (RINO)]
"RINO-undersøkelsen viser at av de tre nevnte praksisene er kjennskap til kollegaers plagiering langt mer utbredt enn kjennskap til kollegaers datafabrikkering eller dataforfalskning."
"Nesten 14 %, eller drøyt 1 av 7 respondenter, oppgir at de kjenner til kollegaers plagiering av andres arbeid de siste tre årene. For fabrikkering eller forfalsking av data er tilsvarende tall kun 2 %, eller 1 av 50."
(Source: https://www.etikkom.no/contentassets/4d637b26fc054f6aa91ac7bbb60ebb92/rino-delrapport-1-2018.pdf) - "Research integrity is defined for these purposes as follows: 1) the research is based on the recognition of fundamental ethical norms such as reliability, truthfulness, respect and accountability; 2) the research follows recognised research ethics regulations and laws; 3) competent use of verifiable and most reproducible methods, and 4) good interplay with quality control mechanisms in research.
All forms of FFP (falsification, fabrication, plagiarism) and QRPs (questionable research practices) are thus regarded as unacceptable. Research integrity aims at high-quality results as an overarching ideal, a high degree of regulatory compliance in the research process, and a high degree of accountability among researchers." (Source: Ibid., p. 6) - "The main findings in the report can be summarised as follows:
- We find a high degree of normative consensus in relation to FFP issues. The clear majority consider such practices to be very problematic.
- The more serious a practice is considered to be, the less common it is for researchers to have either observed colleagues engaging in such practices or for researchers to have engaged in it themselves.
- There is also a normative consensus with regard to QRPs, with a few exceptions, but not to the same extent as for FFP. A higher proportion consider QRPs to be somewhat problematic or not problematic at all.
- A ranking based on the mean assessed severity shows a clear hierarchy, where falsification, fabrication and plagiarism are considered to be the most serious practices, and strategic citation and strategically breaking up results into different publications (salami slicing) to be the least serious.
- A comparison shows some disparities between the disciplines, but these are not extensive. The variation within the disciplines is clearly greater than the variation between the disciplines.
- A majority report having received no training in research ethics, or only one day of training or less. Additionally, only a very small minority report that they have no knowledge of research ethics guidelines or the principles for rightful authorship.
- There is relatively little knowledge on what procedures to be followed when reporting suspected research misconduct." (Source: "New report on research integrity in Norway" Ingrid S. Torp. The Norwegian National Research Ethics Committees. Last updated: 03. July 2018)
- "The RINO survey shows that, of the three mentioned practices, knowledge of colleagues’ plagiarism is far more widespread than knowledge of colleagues fabricating or falsifying data."
- The RINO Project's second report (January 2019) states: 1) over 40 percent of researchers have engaged in at least 1 questionable research practice during the last 3 years and 2) almost 5% of researchers have engaged in at least 3 questionable research practices during the last 3 years. (Source: "40 percent of researchers have "committed" a QRP" Ingrid S. Torp. Last updated: 29. January 2019)
- "The simplest analyses show that a relatively high proportion - just over 40% - of the respondents have committed one or more
questionable research practices over the past three years."
"De enkleste analysene viser at en relativ høy andel – drøyt 40% – av respondentene har begått en eller flere
diskutable praksiser i løpet av de siste tre årene." (Source: "DISKUTABEL FORSKNINGSPRAKSIS: holdninger og handlinger" (Questionable Research Practice: attitudes and actions) Johs. Hjellbrekke, Helene Ingierd, Matthias Kaiser 2019. p. 2. ISBN trykt utgave: 978-82-7682-092-8 ISBN digital utgave: 978-82-7682-091-1)
- "The simplest analyses show that a relatively high proportion - just over 40% - of the respondents have committed one or more
Research Misconduct (continued)
"Research integrity: environment, experience, or ethos?"
The bottom-line, main message of a 2019 Norwegian study of students awarded their PhD degrees at the Faculty of Medicine at the University of Oslo in 2016 states: "Scientific misconduct appears to be an environmental problem as much as an issue of personal integrity."
- "Abstract
Background:
Research integrity has gained attention in the general public as well as in the research community. We wanted to investigate knowledge, attitudes, and practices amongst researchers that have recently finished their PhD and compare this to their responses during their PhD fellowship. In particular, we wanted to investigate whether their attitudes are related to their experiences of their immediate research environment.
Material and method:
Researchers (n = 86) awarded the PhD degree at the Faculty of Medicine at the University of Oslo in 2016 were invited to answer a questionnaire about knowledge, attitudes, and actions related to scientific dishonesty. Seventy-two responded (83.7%). The results were compared with results among first-year doctoral students who responded to the same questionnaire during 2010–2017.
Results:
Overall, 13% of PhDs reported that they knew of people in their immediate research environment who had committed serious forms of scientific dishonesty. A small percentage of PhDs (1.4%) indicated that they themselves had committed such acts. About 3% of the candidates had experienced pressure to commit serious forms of dishonesty and nearly a third of respondents had experienced unethical pressure with respect to authorship during the course of their fellowship. Thirteen percent reported that they had experienced unethical pressure in relation to other forms of dishonesty and 11% had experienced the consequences of some form of scientific dishonesty. Eighteen percent of the respondents believed that one or more actions, which in the literature were perceived as scientific misconduct, were not wrong. We find a connection between attitudes and the perceived research integrity of their research environment. The results also show a difference between PhD students and graduated PhDs in terms of scientific dishonesty. In some areas, the PhDs’ norms are stricter, such as for the use of statistical analysis methods, while there is little change in others, such as in misconduct in order to expedite publications.
Conclusion:
Many PhDs knew about serious forms of scientific misconduct from the research environment in which they are trained, and some also report misconduct themselves. Some experienced pressure to serious forms of misconduct and a large proportion of the respondents had experienced unethical pressure with respect to authorship during their fellowship. Attitudes change during the PhD studies, but ambiguously. Scientific misconduct seems to be an environmental issue as much as a matter of personal integrity.
Main message
- Recently graduated PhDs in biomedicine know of serious forms of scientific misconduct from their research institutions and reveal attitudes that breach with internal norms for scientists. However, few report personal scientific misconduct.
- Many PhDs report unethical pressure with respect to authorship.
- The attitudes of PhD students change during their fellowship, however, in an ambiguous way.
- Scientific misconduct appears to be an environmental problem as much as an issue of personal integrity."
(Source: "Research integrity: environment, experience, or ethos?" Bjørn Hofmann, Søren Holm. Research Ethics Vol 15, Issue 3-4, 2019. First Published October 14, 2019. https://doi.org/10.1177/1747016119880844. URL: https://journals.sagepub.com/doi/full/10.1177/1747016119880844; PDF: https://journals.sagepub.com/doi/pdf/10.1177/1747016119880844) [Note 1: Also see: "Investigating the Reliability and Factor Structure of Kalichman’s “Survey 2: Research Misconduct” Questionnaire: A Post Hoc Analysis Among Biomedical Doctoral Students in Scandinavia" Holm, Søren; Hofmann, Bjørn. Journal of Empirical Research on Human Research Ethics. 2017, 12 (4), 199-205, DOI: http://dx.doi.org/10.1177/1556264617714658] [Note 2: Also see: "
"Unge forskere opplever uetisk press" (Young scientists are experiencing unethical pressure)
- "Rundt tre prosent hadde opplevd press om å fabrikkere, forfalske eller plagiere, såkalt FFP. Dette er de formene for uredelighet som regnes som mest alvorlige. Når det kommer til andre former for uredelighet, øker andelen som bekrefter at de har opplevd press, dramatisk.
– Det mest oppsiktsvekkende gjelder medforfatterskap. Én av tre forteller at de har opplevd press om dette. I tillegg svarer 13 prosent at de har opplevd andre former for uetisk press, for eksempel knyttet til analyse, tolkning eller presentasjon av data, forteller Bjørn Hofmann, som er en av forskerne bak studien."
(Around three percent had experienced pressure to fabricate, falsify or plagiarize, so-called FFP. These are the forms of dishonesty that are considered the most serious. When it comes to other forms of dishonesty, the proportion confirming that they have experienced pressure increases dramatically.
- The most startling thing about co-authorship. One in three reports that they have experienced pressure on this. In addition, 13 percent say that they have experienced other forms of unethical pressure, for example related to analysis, interpretation or presentation of data, says Bjørn Hofmann, one of the researchers behind the study.)
- "Spørsmål om forfatterskap peker seg ut som en stor utfordring også i den landsomfattende studien Research Integrity in Norway (RINO) fra 2018-2019. Mer enn 7000 forskere fra hele landet har svart på spørreundersøkelsen. Nesten ni av ti svarer at de oppfatter såkalt gaveforfatterskap som enten ganske eller svært problematisk. Én av ti vedgår å ha vært med på tildeling av minst ett slikt ufortjent forfatterskap."
(Questions about authorship also prove to be a major challenge in the nationwide study Research Integrity in Norway (RINO) from 2018-2019. More than 7,000 researchers from all over the country have responded to the survey. Nearly nine out of ten respond that they perceive gift authorship as either quite or very problematic. One in ten claims to have been involved in the award of at least one such undeserved authorship.) - – Selv med glimrende forskningsetikkundervisning for PhD-kandidater, vil man kanskje ikke få så stor effekt som man kunne ønske. Rollemodeller, som veiledere og forskningsleder, ser ut til å ha større innflytelse enn oss som driver med forskningsetikk.
(- Even with excellent research ethics education for PhD candidates, you may not get as much impact as you could wish for. Role models, such as supervisors and research leaders, appear to have greater influence than we do in research ethics.) - Lohne og Elken mener flere av funnene peker på et institusjonelt ansvar – i form av opplæring og tilstrekkelig infrastruktur for å følge opp problematiske saker.
(Lohne and Elken believe that several of the findings point to institutional responsibility - in the form of training and sufficient infrastructure to follow up on problematic issues.)
(Source: "Unge forskere opplever uetisk press" (Young scientists are experiencing unethical pressure) in Forsknings etikk, Et magasin fra De nasjonale forskningsetiske komiteene, NR. 4 • Desember 2019 • 19. årgang, Brukermedvirkning: Ekspertenes ekspert, p. 4-6. PDF: https://www.etikkom.no/globalassets/documents/bladet-forskningsetikk/alle-utgaver/48630_forskningsetikk_nr4_2019_low_dobbelsider.pdf) [Note: Also see URL: https://www.etikkom.no/Aktuelt/Fagbladet-Forskningsetikk/arkiv/2019/2019-4/unge-forskere-opplever-uetisk-press/]
Research Misconduct (continued)
"A new Act on Research Ethics/Integrity in Norway"
"A new Act on Research Ethics/Integrity in Norway"
- "The first Act on Research Integrity came into effect in 2007. It was very much focused on establishing and regulating “The National Commission for Dealing with Research Misconduct” although the main responsibility rested at the local research institutions.
The new Act came into effect 1 May 2017. The main purpose is to hold researchers and research institutions responsible for good scientific practice. In short researchers are made responsible for knowing relevant guidelines i.e. how to be a responsible researcher and how to avoid research misconduct.
Research Institutions must ensure that research at the Institution is conducted according to recognized “research ethical norms”. Each research institution is responsible for training of young or senior researchers and for ensuring that all its researchers know about relevant norms.
Research institutions must have procedures for dealing with breaches and must establish “Research Ethics/Research Integrity Committees”. The research institutions must deal with research misconduct (defined in the law as fabrication., falsification, plagiarism and other serious breaches committed intentionally or by gross negligence) and less serious breaches/questionable research practices.
The National Commission is independent and meant as a guiding resource for the institutions. The institutions must inform the National Commission about the outcome of investigations of possible research misconduct. The Commission may react in different ways or just approve. The National Commission has thus a kind of “oversight”. Further the National Commission may deal with any possible serious case on its own initiative even if the case has already been dealt with at the local level.
If a local investigation concludes with research misconduct, the alleged person may refer the case to the National Commission as an appeal body.
For more than 25 years there have been National Research Ethics/Integrity Committees covering different fields. The three committees are primarily advisory bodies and are issuing national guidelines. The Act specifies the role and responsibility for these committees.
For more information please visit the website: www.etikkom.no" (Source: "A new Act on Research Ethics/Integrity in Norway" European Network of Research Integrity Offices (ENRIO) website: http://www.enrio.eu/news-activities/new-act-research-ethicsintegrity-norway/)
Research Misconduct (continued)
"They did a bad bad thing"
When it comes to research misconduct, burying one's head in the sand and pretending it doesn't exist is the worst possible plan.
Department of Health and Human Service, Office of Research Integrity
Research Misconduct (continued)
"CHECKLIST: Policies and Procedures for Handling Research Misconduct Allegations"
"This checklist is used by the U.S. Department of Health and Human Services, Office of Research Integrity (ORI) and is intended only to provide general information regarding ORI’s review of institutional policies. " (Source: "CHECKLIST: Policies and Procedures for Handling Research Misconduct Allegations" Last Modified: 04/05/2017. U.S. Office of Research Integrity)
"How to investigate allegations of research misconduct: A checklist"
"Do investigations into research misconduct allegations need better standards? The Association for the Promotion of Research Integrity (APRIN) in Japan, a group of volunteers who “commit themselves to the promotion of research of high integrity” and provide “e-learning material for research ethics education,” thinks so. Today, we present a guest post by Iekuni Ichikawa, who chaired an APRIN committee that recently came up with a new checklist for such investigations, about the effort. (Source: "How to investigate allegations of research misconduct: A checklist" Iekuni Ichikawa. Retraction Watch Posted on January 8, 2019; Also see: "Checklist for Investigating Allegations of Research Misconduct")
"Investigation of Allegations of Research Misconduct"
"Research misconduct is very narrowly defined as: (1) Fabrication of results, making up data; (2) Falsification of data, manipulating research materials, equipment or processes, or changing or omitting data or results such that the research is not accurately represented in the research record or; (3) Plagiarism, the appropriation of another person’s ideas, processes, results or words without giving credit.
Maintaining the highest ethical standards in the conduct of research remains the goal of the entire scientific community at the NIH. Our good reputation is essential for maintaining the trust of Congress, the American People, our Scientific Colleagues across the US and the International Community. It is the responsibility of each Investigator who participates in research at the NIH, no matter what their career stage, to maintain exemplary standard of intellectual honesty in formulating or conducting research and to report any suspected research misconduct to the NIH Agency Research Integrity Officer (ARIO)."
Research Misconduct (continued)
U.S. National Science Foundation (NSF)
Included below is the formal definition of research misconduct by the U.S. National Science Foundation (NSF), the largest funding organization of research and education in most fields of science and engineering.
(a) Research misconduct means fabrication, falsification, or plagiarism in proposing or performing research funded by NSF, reviewing research proposals submitted to NSF, or in reporting research results funded by NSF.
Research Misconduct (continued)
U.S. Federal Register, Part III, Department of Health and Human Services
CFR › Title 42 › Chapter I › Subchapter H › Part 93 › Subpart C › Section 93.310
"§ 93.310 Institutional investigation.
Institutions conducting research misconduct investigations must:
§ 93.103 Research misconduct.
Research misconduct means fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
Research Misconduct (continued)
Stanford University
Research Policy Handbook
1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting
3. Definitions
"A. Research Misconduct
"Research misconduct" is defined as fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
A finding of research misconduct requires that:
"Understanding Stanford’s Code of Conduct"
Research Misconduct (continued)
The International Association of Scientific, Technical and Medical Publishers (STM)
"The STM Report: An overview of scientific and scholarly publishing, Fifth edition, October 2018"
"Scientific Misconduct and Medical Journals"
"Duke University to settle case alleging researchers used fraudulent data to win millions in grants"
"UK universities fall short on reporting misconduct investigations"
"Parliamentary inquiry recommends creation of research integrity committee."
Umbrella body for research councils accepts MPs’ call for organisation to monitor whether universities carry out misconduct investigations properly
Research Misconduct (continued)
"It’s time to end the code of silence at universities"
"Years of Ethics Charges, but Star Cancer Researcher Gets a Pass"
"Institutional Research Misconduct Reports Need More Credibility"
Research Misconduct (continued)
"Quality of reports of investigations of research integrity by academic institutions"
"What universities can learn from one of science’s biggest frauds"
"Detailed analysis of misconduct investigations into huge research fraud suggests institutional probes aren’t rigorous enough."
"Stanford University's Research Policy HandbooK (RPH)"
"1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting"
Presents procedures for reporting and investigating allegations of research misconduct, and for the required notifications to federal agencies of such allegations and investigations.
"Lies, Damned Lies, and Medical Science"
"Dr. John Ioannidis Exposes the Bad Science of Colleagues - The Atlantic"
The Stanford Center for Clinical and Translational Research and Education, Stanford University
Meta-Research Innovation Center, Stanford University
Best Practices in Science (BPS Group), Stanford University
Research Misconduct (continued)
"To catch misconduct, journals are hiring research integrity czars"
"China introduces sweeping reforms to crack down on academic misconduct"
"Initiatives include a national list of cases, a blacklist of ‘poor quality’ journals and a government agency that is responsible for policing misconduct."
Research Misconduct (continued)
The Norwegian National Research Ethics Committees
Research Misconduct (continued)
Research Integrity in Norway (RINO) – a study of scientific misconduct and questionable research practices
"They did a bad bad thing"
When it comes to research misconduct, burying one's head in the sand and pretending it doesn't exist is the worst possible plan.
- "With human nature as it is, the only surprising thing about scientific misconduct should be that it continues to surprise us. After all, scientists are human — with the same range of creativity, hopes, fears, anxieties and weaknesses as anyone else. Why should we be more surprised when scientists behave unethically than, say, those in business or politics? Surprised or not, we should acknowledge that scientific misconduct is happening, will always happen, and probably always has happened. With an increased awareness, however, we can all be more vigilant and perhaps better equipped to prevent it happening." (Source: "They did a bad bad thing" Editorial. Nature Chemistry volume 3, page 337 (2011) Published 20 April 2011 DOI https://doi.org/10.1038/nchem.1042)
Department of Health and Human Service, Office of Research Integrity
- Definition of Research Misconduct
Research misconduct means fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
(a) Fabrication is making up data or results and recording or reporting them.
(b) Falsification is manipulating research materials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented in the research record.
(c) Plagiarism is the appropriation of another person's ideas, processes, results, or words without giving appropriate credit.
(d) Research misconduct does not include honest error or differences of opinion.
(Source: U.S. Department of Health and Human Service, Office of Research Integrity. https://ori.hhs.gov/definition-misconduct) - "Avoiding Plagiarism, Self-plagiarism, and Other Questionable Writing Practices: A Guide to Ethical Writing"
"The purpose of this module is to help students, as well as professionals, identify and prevent questionable practices and to develop an awareness of ethical writing. This guide was written by Miguel Roig, PhD, from St. Johns University with funding from ORI."
Research Misconduct (continued)
"CHECKLIST: Policies and Procedures for Handling Research Misconduct Allegations"
"This checklist is used by the U.S. Department of Health and Human Services, Office of Research Integrity (ORI) and is intended only to provide general information regarding ORI’s review of institutional policies. " (Source: "CHECKLIST: Policies and Procedures for Handling Research Misconduct Allegations" Last Modified: 04/05/2017. U.S. Office of Research Integrity)
"How to investigate allegations of research misconduct: A checklist"
"Do investigations into research misconduct allegations need better standards? The Association for the Promotion of Research Integrity (APRIN) in Japan, a group of volunteers who “commit themselves to the promotion of research of high integrity” and provide “e-learning material for research ethics education,” thinks so. Today, we present a guest post by Iekuni Ichikawa, who chaired an APRIN committee that recently came up with a new checklist for such investigations, about the effort. (Source: "How to investigate allegations of research misconduct: A checklist" Iekuni Ichikawa. Retraction Watch Posted on January 8, 2019; Also see: "Checklist for Investigating Allegations of Research Misconduct")
"Investigation of Allegations of Research Misconduct"
"Research misconduct is very narrowly defined as: (1) Fabrication of results, making up data; (2) Falsification of data, manipulating research materials, equipment or processes, or changing or omitting data or results such that the research is not accurately represented in the research record or; (3) Plagiarism, the appropriation of another person’s ideas, processes, results or words without giving credit.
Maintaining the highest ethical standards in the conduct of research remains the goal of the entire scientific community at the NIH. Our good reputation is essential for maintaining the trust of Congress, the American People, our Scientific Colleagues across the US and the International Community. It is the responsibility of each Investigator who participates in research at the NIH, no matter what their career stage, to maintain exemplary standard of intellectual honesty in formulating or conducting research and to report any suspected research misconduct to the NIH Agency Research Integrity Officer (ARIO)."
- NIH IRP Policies & Procedures for Research Misconduct Proceedings (PDF File)
- Guide to Handling of Research Misconduct Allegations (PDF File)
- Public Health Service Policies on Research Misconduct (PDF File)
(Source: "Investigation of Allegations of Research Misconduct" National Institutes of Health, Office of Intramural Research)
Research Misconduct (continued)
U.S. National Science Foundation (NSF)
Included below is the formal definition of research misconduct by the U.S. National Science Foundation (NSF), the largest funding organization of research and education in most fields of science and engineering.
(a) Research misconduct means fabrication, falsification, or plagiarism in proposing or performing research funded by NSF, reviewing research proposals submitted to NSF, or in reporting research results funded by NSF.
- Fabrication means making up data or results and recording or reporting them.
- Falsification means manipulating research materials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented in the research record.
- Plagiarism means the appropriation of another person’s ideas, processes, results or words without giving appropriate credit.
Research Misconduct (continued)
U.S. Federal Register, Part III, Department of Health and Human Services
CFR › Title 42 › Chapter I › Subchapter H › Part 93 › Subpart C › Section 93.310
"§ 93.310 Institutional investigation.
Institutions conducting research misconduct investigations must:
- (a) Time. Begin the investigation within 30 days after determining that an investigation is warranted.
- (b) Notice to ORI. Notify the ORI Director of the decision to begin an investigation on or before the date the investigation begins and provide an inquiry report that meets the requirements of § 93.307 and § 93.309.
- (c) Notice to the respondent. Notify the respondent in writing of the allegations within a reasonable amount of time after determining that an investigation is warranted, but before the investigation begins. The institution must give the respondent written notice of any new allegations of research misconduct within a reasonable amount of time of deciding to pursue allegations not addressed during the inquiry or in the initial notice of investigation.
- (d) Custody of the records. To the extent they have not already done so at the allegation or inquiry stages, take all reasonable and practical steps to obtain custody of all the research records and evidence needed to conduct the research misconduct proceeding, inventory the records and evidence, and sequester them in a secure manner, except that where the research records or evidence encompass scientific instruments shared by a number of users, custody may be limited to copies of the data or evidence on such instruments, so long as those copies are substantially equivalent to the evidentiary value of the instruments. Whenever possible, the institution must take custody of the records -
(1) Before or at the time the institution notifies the respondent; and
(2) Whenever additional items become known or relevant to the investigation. - (e) Documentation. Use diligent efforts to ensure that the investigation is thorough and sufficiently documented and includes examination of all research records and evidence relevant to reaching a decision on the merits of the allegations.
- (f) Ensuring a fair investigation. Take reasonable steps to ensure an impartial and unbiased investigation to the maximum extent practicable, including participation of persons with appropriate scientific expertise who do not have unresolved personal, professional, or financial conflicts of interest with those involved with the inquiry or investigation.
- (g) Interviews. Interview each respondent, complainant, and any other available person who has been reasonably identified as having information regarding any relevant aspects of the investigation, including witnesses identified by the respondent, and record or transcribe each interview, provide the recording or transcript to the interviewee for correction, and include the recording or transcript in the record of the investigation.
(h) Pursue leads. Pursue diligently all significant issues and leads discovered that are determined relevant to the investigation, including any evidence of additional instances of possible research misconduct, and continue the investigation to completion.
(Source: Cornell University, Cornell Law School, Legal Information Institute (LII) "42 CFR 93.310 - Institutional investigation." Also see: "42 CFR Part 93 - PUBLIC HEALTH SERVICE POLICIES ON RESEARCH MISCONDUCT" Also see: Part III, Department of Health and Human Services, 42 CFR Parts 50 and 93 Public Health Service Policies on Research Misconduct; Final Rule, Federal Register / Vol. 70, No. 94 / Tuesday, May 17, 2005 / Rules and Regulations) - Also see: Cornell Law School, Legal Information Institute
42 CFR Part 93 - PUBLIC HEALTH SERVICE POLICIES ON RESEARCH MISCONDUCT
https://www.law.cornell.edu/cfr/text/42/part-93
§ 93.103 Research misconduct.
Research misconduct means fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
- (a) Fabrication is making up data or results and recording or reporting them.
- (b) Falsification is manipulating researchmaterials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented in the research record.
- (c) Plagiarism is the appropriation of anotherperson's ideas, processes, results, or words without giving appropriate credit.
- (d) Research misconduct does not include honest error or differences of opinion.
(Source: Cornell University, Cornell Law School, Legal Information Institute (LII) "42 CFR 93.103 - Research misconduct.")
Research Misconduct (continued)
Stanford University
Research Policy Handbook
1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting
3. Definitions
"A. Research Misconduct
"Research misconduct" is defined as fabrication, falsification, or plagiarism in proposing, performing, or reviewing research, or in reporting research results.
- Fabrication means making up data or results, and recording or reporting them.
- Falsification means manipulating research materials, equipment, or processes, or changing or omitting data or results such that the research is not accurately represented in the research record.
- Plagiarism means the appropriation of another person's ideas, processes, results, or words without giving appropriate credit.
A finding of research misconduct requires that:
- there is a significant departure from accepted practices of the relevant research community
- the misconduct is committed intentionally, or knowingly, or recklessly
- the allegation is proven by a preponderance of the evidence. (Stanford University's disciplinary procedures may establish a different standard of proof for disciplinary actions
(Source: https://doresearch.stanford.edu/policies/research-policy-handbook/conduct-research/research-misconduct-policy-allegations-investigations-and-reporting#main-content)
"Understanding Stanford’s Code of Conduct"
- "The code is a shared statement of goals, rules and values designed to build trust, protect members of the university community and help people act with integrity. It applies to a broad group, ranging from faculty, staff, students and trustees to consultants, contractors and volunteers. Members of the university community can use the code as a guide to help identify and avoid problem areas, such as workplace, scientific and research misconduct; discrimination and harassment; conflicts of interest or commitment; privacy issues; and fraud, theft and embezzlement."
(...)
"Each of us is expected to act in accordance with the law and with university policies and procedures. As a member of the community, you are expected to raise concerns or report suspected violations of the code, even when these concerns or violations may not affect you directly. It is important to note that we consider this a service to the institution and have a strong non-retaliation policy for those who, in good faith, report or provide information about concerns or suspected violations.
The code supports our mission and vision by reinforcing the fact that we are each accountable for our own actions. As members of the university community, we are collectively accountable for upholding these standards of behavior, as well as for compliance with all applicable laws, regulations and policies that guide our work.
Stanford has high ideals. The code helps us live up to them and support the expectations that accompany them." (Source: "Understanding Stanford’s Code of Conduct" "Anne Sweeney Hoy, Stanford’s chief ethics and compliance officer, discusses the Code of Conduct, which is a tool kit for upholding Stanford’s high ethical standards." BY CHRIS PEACOCK. Stanford University News DECEMBER 11, 2018)
Research Misconduct (continued)
The International Association of Scientific, Technical and Medical Publishers (STM)
"The STM Report: An overview of scientific and scholarly publishing, Fifth edition, October 2018"
- "2.15 Publishing ethics
There has been a growing awareness of the need for higher (or at least more transparent) ethical standards in journal publishing to deal with issues such as conflict of interest, ghostwriting, guest authorship, citation rings, peer review rigging, authorship disputes, falsification and fabrication of data, scientific fraud, unethical experimentation and plagiarism. Much of the criticism has been addressed at the intersection of the biomedical journals and pharmaceutical industry but the issues are by no means unique to this sector." (Source: "The STM Report: An overview of scientific and scholarly publishing, Fifth edition, October 2018" Rob Johnson, Research Consulting; Anthony Watkinson, CIBER Research; Michael Mabe, The International Association of Scientific, Technical and Medical Publishers.
"Scientific Misconduct and Medical Journals"
- "The Role and Responsibilities of Institutions
Institutions are expected to conduct an appropriate and thorough investigation of allegations of scientific misconduct. Some institutions are immediately responsive, acknowledging receipt of the letter from the journal describing the concerns, and quickly begin an investigation. In other cases, it may take time to identify the appropriate institutional individuals to contact, and even then, many months to receive a response. Some institutions appear well-equipped to conduct investigations, whereas other institutions appear to have little experience in such matters or fail to conduct adequate investigations 13; these institutions can take months to years to provide JAMA with an adequate response. In some cases involving questions of misconduct from outside of the United States, institutions have indicated that further investigation must wait until numerous legal issues are resolved, further delaying a response." (Source: "Scientific Misconduct and Medical Journals" Howard Bauchner, MD; Phil B. Fontanarosa, MD, MBA; Annette Flanagin, RN, MA; Joe Thornton, JD. Editorial, JAMA. Published online October 19, 2018. doi:10.1001/jama.2018.14350)
- "Duke has been hit by multiple high-profile misconduct cases in recent years. One such case involved Anil Potti, a once-rising star in cancer research who fabricated data, leading to 12 retractions, multiple lawsuits, and reprimands from the medical board. The university is also involved in an ongoing lawsuit filed by a whistleblower, which alleges that a pulmonary scientist, her supervisor, and the university included fraudulent data in federal documents associated with more dozens of grants worth hundreds of millions of dollars." (Source: "Duke’s mishandling of misconduct prompts new U.S. government grant oversight" By Alison McCook, Retraction Watch in Science Mar. 23, 2018 , 4:15 PM) [Note: See: "Case Summary: Potti, Anil" and "Notice is hereby given that the Office of Research Integrity (ORI) has taken final action in the following case: Anil Potti, M.D., Duke University School of Medicine"]
"Duke University to settle case alleging researchers used fraudulent data to win millions in grants"
- "According to court documents filed last week in the Middle District of North Carolina in Greensboro, former Duke biologist Joseph Thomas, who sued the university in 2015 under a federal law that allows whistleblowers to receive as much as 30% of any payout, is waiting for the U.S. Department of Justice to approve the settlement. Thomas brought his case under the federal False Claims Act (FCA), which could force Duke to return to the government up to three times the amount of any ill-gotten funds." (Source: "Duke University to settle case alleging researchers used fraudulent data to win millions in grants" By Ivan Oransky, Retraction Watch Nov. 19, 2018, 12:10 PM. Science. Posted in: Scientific Community. doi:10.1126/science.aaw1185)
"UK universities fall short on reporting misconduct investigations"
"Parliamentary inquiry recommends creation of research integrity committee."
- “Establishing a new national Research Integrity Committee is crucial,” says committee chairman and member of parliament, Norman Lamb. “It’s not a good look for the research community to be dragging its heels on this, particularly given research fraud can quite literally become a matter of life and death.” (Source: "UK universities fall short on reporting misconduct investigations" Nature NEWS 11 JULY 2018. doi: 10.1038/d41586-018-05697-7)
- "For the latest inquiry, the UK's Science and Technology Committee — on which sit 11 members of parliament — contacted 136 UK universities to ask whether they publish information about the number of research misconduct investigations they conduct annually. Around 25% said they didn’t, the report says, with some citing concern that such transparency could undermine their public image. The report says there is a need to address the potential conflicts faced by universities' self-policing in the current system." (Source: Ibid.)
- "The lack of compliance by universities, despite it being a prerequisite for eligibility for grants from major funders, might be partially attributable to the lack of penalties imposed, the report notes." (Source: Ibid.)
- "The report recommends that universities, funders and publishers should share information about misconduct inquiries. An investigation by the BBC last year found that at least 300 allegations of research misconduct had been reported at 23 out of 24 Russell Group universities between 2011 and 2016. Around a third of allegations of plagiarism, fabrication, piracy and misconduct were upheld, the BBC reported, and more than 30 studies were retracted." (Source: Ibid.)
- "2. Problems with the integrity of research—when research is anything less than “rigorous, accurate, honest and transparent”2—can arise from errors, poor research design, or even outright fraud. All of these problems have the potential to tarnish the reputation of research, and erroneous deductions and conclusions can have dramatic consequences, particularly in the context of medical research." (Source: "Research integrity Sixth Report of Session 2017–19" Report, together with formal minutes relating to the report, House of Commons Science and Technology Committee. HC 350, Published on 11 July 2018 by authority of the House of Commons. p. 5)
Umbrella body for research councils accepts MPs’ call for organisation to monitor whether universities carry out misconduct investigations properly
- "A UKRI spokesman said: “We welcomed the recommendation by the Science and Technology Committee on the creation of a new research integrity committee and we are actively working on its development alongside government and key stakeholders. We will provide an update in summer 2019.” (Source: "UKRI agrees to create research integrity watchdog" Chris Havergal. Times Higher Education 10 JUNE 2019)
Research Misconduct (continued)
"It’s time to end the code of silence at universities"
- "Although two Federal agencies — the U.S. Office of Research Integrity and the National Science Foundation’s Office of the Inspector General — do have oversight of university investigations into scientific misconduct, the public has no way of knowing whether such investigations were carried out properly. As we and a colleague wrote in the Journal of the American Medical Association earlier this year, when universities investigate their own — which by Federal statute, they are obligated to do — they have serious conflicts of interest, and the resulting investigations are often deeply flawed." (Source: "It’s time to end the code of silence at universities" Ivan Oransky. Retraction Watch November 6, 2018)
- "Bulgarian university rector faces dismissal over plagiarism"
"Bulgaria’s Education Minister will sack the rector of the Technical University of Varna, Rossen Vassilev, after an ethics commission found evidence of extensive plagiarism in his doctoral dissertation.
It is a first for Bulgaria, after amendments passed earlier this year created the legal grounds for an academic ethics commission at the Education Ministry. The amendments were, in part, prompted by the highly-publicised accusations against Vassilev, which first surfaced last year and made by some of colleagues at the university.
At the time, the law’s provisions only allowed for a court challenge from the plagiarised authors.
Under the amended law, Vassilev’s doctoral dissertation and scientific publications he presented to be granted the professor title were reviewed by three arbiters. Their findings – namely that Vassilev plagiarised more than 90 per cent of his dissertation, including spelling and grammar mistakes in the source material – were the grounds for the ethics commission to rule that Vassilev was guilty of plagiarism.
Bulgaria’s Education Minister Krassimir Vulchev said that Vassilev will be stripped of this doctoral degree, would lose his professor title and the position as rector of the university. An interim rector would be appointed by the minister next week, who would terminate Vassilev’s employment contract.
But the law does not prevent Vassilev from retaining his position as a lecturer at the university – docent, in Bulgarian academia parlance – and could even be re-elected rector by the university’s general assembly, one media report claimed.
Vassilev previously denied the accusations against him and the Technical University of Varna published a lengthy rebuttal of the ethics committee’s decision, questioning the motivation of Vassilev’s accusers and the credentials of the arbiters selected to review his dissertation.
It also warned that the ethics commission was being used for a “witch hunt” and said that any disputes about plagiarism should be settled in court." (Source: "Written by The Sofia Globe staff on November 9, 2018 in Bulgaria - Comments Off on Bulgarian university rector faces dismissal over plagiarism")
"Years of Ethics Charges, but Star Cancer Researcher Gets a Pass"
- "'A Tremendous Conflict of Interest'
Within the realm of biomedical science, it falls to the Office of Research Integrity to issue formal findings of scientific misconduct, which can lead to suspension of federal financing and effectively end a research career. The office labors under an awkward constraint: It does not carry out its own investigations, but relies on accused researchers’ own institutions to forward their findings.
With their own reputations on the line, institutions “have a tremendous conflict of interest,” said Dr. Richard Smith, former editor of The British Medical Journal and a founding member of the Committee on Publication Ethics in Britain. “There’s a terrible temptation to bury it all,” he added.
There are also dollars at stake. Of the $29.1 million Dr. Croce has received in federal funding as a principal investigator while at Ohio State, university records show, $8.7 million has gone directly to the university in overhead payments, a fairly standard cut for research institutions." (Source: "Years of Ethics Charges, but Star Cancer Researcher Gets a Pass" By James Glanz and Agustin Armendariz. The New York Times March 8, 2017. "A version of this article appears in print on March 9, 2017, on Page A1 of the New York edition with the headline: Years of Questions, but Researcher Gets a Pass.") [Also see: "Judge dismisses most of Carlo Croce’s libel case against the New York Times" in Retraction Watch. Ivan Oransky. November 8, 2018]
"Institutional Research Misconduct Reports Need More Credibility"
- "As a result, like most elements of research misconduct, little is known about institutions’ responses to potential misconduct by their own members. The community that relies on the integrity of university research does not have access to information about how often such claims arise, or how they are resolved. Nonetheless, there are some indications that many internal reviews are deficient.
(...)
The Office of Inspector General (OIG) of the National Science Foundation (NSF) has found that the reports of some institutions do not meet reasonable standards. For instance, some reports from universities do not ask relevant research questions, or they fail to appropriately expand the investigation beyond a particular allegation; other reports focus on finding fault with an individual when many were involved in the research; and for some other reports, committee members have lacked relevant expertise (email from Alan Boehm, MFS; James T. Kroll, PhD; and Aaron S. Manka, PhD; December 2017). These are not idiosyncratic or 1-time problems. A partial list of shortcomings that the OIG staff has compiled and shared at conferences includes the following:- Investigative reports that lack supporting evidence and fail to address the elements of a research misconduct finding, particularly intent;
- Individuals who are the subjects of the investigation blaming the student or postdoctoral researchers, but the investigative committee never interviewing these individuals;
- Accepting, without question, excuses by the subjects of the investigation; and
- Relying only on information in allegations, not checking for patterns or other misconduct."
- "The group developed a proposed checklist for research integrity investigation (eAppendix in the Supplement). The checklist, or its improved successors, is designed to address whether an investigation follows reasonable standards and if the subsequent report is appropriate and complete. For example, it addresses the following issues:
- Whether a specific institutional investigation plan or report is generated that identifies appropriate questions to pursue and proposes a meaningful approach to securing the answers;
- Whether the correct individuals are interviewed;
- Whether the relevant data are secured and reviewed by appropriate experts;
- Whether the report provides factual basis and data; and
- Whether the report supports its conclusions." (Source: "Institutional Research Misconduct Reports Need More Credibility" C. K. Gunsalus, JD; Adam R. Marcus, MA; Ivan Oransky, MD. JAMA. 2018;319(13):1315-1316. doi:10.1001/jama.2018.0358)
Research Misconduct (continued)
"Quality of reports of investigations of research integrity by academic institutions"
- "Conclusions
Our analyses identify important deficiencies in the quality and reporting of institutional investigation of concerns about research integrity. They reinforce disquiet about the ability of institutions to rigorously and objectively oversee the integrity of research conducted by their own employees and the lack of regulatory oversight. A possible solution is the establishment of more efficient and adequately resourced independent organisations with the authority and expertise to undertake and report investigations, and implement recommendations, including those which span multiple institutions and countries." (Source: "Quality of reports of investigations of research integrity by academic institutions" Andrew Grey, Mark Bolland, Greg Gamble and Alison Avenell, Research Integrity and Peer Review 2019 4:3 https://doi.org/10.1186/s41073-019-0062-x © The Author(s) 2019. Received: 9 December 2018, Accepted: 5 February 2019, Published: 19 February 2019)
"What universities can learn from one of science’s biggest frauds"
"Detailed analysis of misconduct investigations into huge research fraud suggests institutional probes aren’t rigorous enough."
- "At the World Conference on Research Integrity in Hong Kong from 2 to 5 June, Grey’s team described its years-long efforts to clean up Sato’s literature, and presented its analysis of the inquiries conducted by four universities in Japan and the United States ensnared in the scandal (the team published its analysis of three investigations in a paper in February1). Grey says their findings provide evidence to support a growing view in the academic community: that university investigations into research misconduct are often inadequate, opaque and poorly conducted. They challenge the idea that institutions can police themselves on research integrity and propose that there should be independent organizations to evaluate allegations of research fraud." (Source: "What universities can learn from one of science’s biggest frauds" Holly Else, Nature 570, 287-288, p. 287 (2019) doi: 10.1038/d41586-019-01884-2)
- "The analysis is one of just a few to look closely at research-misconduct investigations, and the first to use a systematic approach to rate them, says C. K. Gunsalus, a specialist in research integrity at the University of Illinois at Urbana–Champaign, who was not part of the analysis. Too many research-misconduct investigations turn out to be inadequate or flawed, says Gunsalus. She had a hand in creating a 26-point checklist that university officials can use to guide probes into research misconduct; Grey’s team used this to rate the investigations." (Source: Ibid., p. 287)
- "Grey’s team rated each report as inadequate overall. The researchers also suggest that the investigations focused too much on determining whether research misconduct had occurred, rather than on understanding the validity of the research in question and correcting or retracting unreliable articles. Grey and his colleagues argue that protecting the integrity of the literature should be the priority of any investigation — because integrity can be compromised without evidence of misconduct." (Source: Ibid., p. 288)
- "Gunsalus agrees that the Sato case highlights some of the problems with misconduct investigations, and says that if shortcomings emerge, further reviews might be needed. She suggests that institutional panels should include external members, and that officials should use a standardized checklist to strengthen their processes. “There should be some way for journals, funders, patients and others to be assured of the credibility and thoroughness of university reviews,” she says." (Source: Ibid., p. 288)
"Stanford University's Research Policy HandbooK (RPH)"
- Policies
"1. Conduct of Research
1.1 Principles Concerning Research
1.2 Rights and Responsibilities in the Conduct of Research
1.3 Academic Freedom
1.4 Openness in Research
1.5 On Academic Authorship
1.6 Multi-Authored Research Papers
1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting
1.8 Nondiscrimination in Research Agreements
1.9 Retention of and Access to Research Data"
(Source: https://doresearch.stanford.edu/policies/research-policy-handbook)
"1.7 Research Misconduct: Policy on Allegations, Investigations, and Reporting"
Presents procedures for reporting and investigating allegations of research misconduct, and for the required notifications to federal agencies of such allegations and investigations.
- Jump To:
1. Introduction
2. Applicability
3. Definitions
4. Federal Funding Agency Requirements
5. Individual Reporting Responsibility
6. Procedure for School Dean's Review
7. Internal Coordination/Reports to the Dean of Research
8. Notification to External Agencies
9. Determination of Discipline
10. Cautions and Assistance"(Source: https://doresearch.stanford.edu/policies/research-policy-handbook/conduct-research/research-misconduct-policy-allegations-investigations-and-reporting#main-content)
"Lies, Damned Lies, and Medical Science"
"Dr. John Ioannidis Exposes the Bad Science of Colleagues - The Atlantic"
- "Much of what medical researchers conclude in their studies is misleading, exaggerated, or flat-out wrong. So why are doctors—to a striking extent—still drawing upon misinformation in their everyday practice? Dr. John Ioannidis has spent his career challenging his peers by exposing their bad science.
(...)
But beyond the headlines, Ioannidis was shocked at the range and reach of the reversals he was seeing in everyday medical research. “Randomized controlled trials,” which compare how one group responds to a treatment against how an identical group fares without the treatment, had long been considered nearly unshakable evidence, but they, too, ended up being wrong some of the time. “I realized even our gold-standard research had a lot of problems,” he says. Baffled, he started looking for the specific ways in which studies were going wrong. And before long he discovered that the range of errors being committed was astonishing: from what questions researchers posed, to how they set up the studies, to which patients they recruited for the studies, to which measurements they took, to how they analyzed the data, to how they presented their results, to how particular studies came to be published in medical journals.
This array suggested a bigger, underlying dysfunction, and Ioannidis thought he knew what it was. “The studies were biased,” he says. “Sometimes they were overtly biased. Sometimes it was difficult to see the bias, but it was there.” Researchers headed into their studies wanting certain results—and, lo and behold, they were getting them. We think of the scientific process as being objective, rigorous, and even ruthless in separating out what is true from what we merely wish to be true, but in fact it’s easy to manipulate results, even unintentionally or unconsciously. “At every step in the process, there is room to distort results, a way to make a stronger claim or to select what is going to be concluded,” says Ioannidis. “There is an intellectual conflict of interest that pressures researchers to find whatever it is that is most likely to get them funded.
(...)
THOUGH SCIENTISTS AND science journalists are constantly talking up the value of the peer-review process, researchers admit among themselves that biased, erroneous, and even blatantly fraudulent studies easily slip through it. Nature, the grande dame of science journals, stated in a 2006 editorial, “Scientists understand that peer review per se provides only a minimal assurance of quality, and that the public conception of peer review as a stamp of authentication is far from the truth.” What’s more, the peer-review process often pressures researchers to shy away from striking out in genuinely new directions, and instead to build on the findings of their colleagues (that is, their potential reviewers) in ways that only seem like breakthroughs—as with the exciting-sounding gene linkages (autism genes identified!) and nutritional findings (olive oil lowers blood pressure!) that are really just dubious and conflicting variations on a theme.
(...)
Perhaps only a minority of researchers were succumbing to this bias, but their distorted findings were having an outsize effect on published research. To get funding and tenured positions, and often merely to stay afloat, researchers have to get their work published in well-regarded journals, where rejection rates can climb above 90 percent. Not surprisingly, the studies that tend to make the grade are those with eye-catching findings. But while coming up with eye-catching theories is relatively easy, getting reality to bear them out is another matter. The great majority collapse under the weight of contradictory data when studied rigorously. Imagine, though, that five different research teams test an interesting theory that’s making the rounds, and four of the groups correctly prove the idea false, while the one less cautious group incorrectly “proves” it true through some combination of error, fluke, and clever selection of data. Guess whose findings your doctor ends up reading about in the journal, and you end up hearing about on the evening news? Researchers can sometimes win attention by refuting a prominent finding, which can help to at least raise doubts about results, but in general it is far more rewarding to add a new insight or exciting-sounding twist to existing research than to retest its basic premises—after all, simply re-proving someone else’s results is unlikely to get you published, and attempting to undermine the work of respected colleagues can have ugly professional repercussions." (Source: "Lies, Damned Lies, and Medical Science" David H. Freedman. The Atlantic November 2010 Issue)
The Stanford Center for Clinical and Translational Research and Education, Stanford University
- The mission of Spectrum, Stanford’s Clinical and Translational Science Award (CTSA)-supported research hub, is to transform clinical and translational research and education at Stanford to make it more effective at discovering and implementing data-driven strategies to serve the health needs of individuals and the population. Spectrum’s programs extend from the earliest stages of the translational pipeline to the “final mile” of implementation science at the patient and population levels. Spectrum leverages local achievements, institutional strengths and resources, and advancements in the focus of the CTSA consortium to nurture an innovative and collaborative workforce that can transfer health discoveries from the University to patients and the community.
Meta-Research Innovation Center, Stanford University
- Goals
- Build the meta-research field and catalyze solutions-focused research to develop best scientific practices
- Provide leaders with the skills and knowledge needed to support the development and implementation of high-quality research
- Transform research practices to strengthen the evidence base for informed decision-making
Best Practices in Science (BPS Group), Stanford University
Research Misconduct (continued)
"To catch misconduct, journals are hiring research integrity czars"
- "Scientific journals’ creation of dedicated positions for rooting out misconduct before publication comes amid growing awareness of such issues, and stems from a recognition that spot-checking and other ad hoc arrangements were insufficient" (Source: "To catch misconduct, journals are hiring research integrity czars" By IVAN ORANSKY and ADAM MARCUS. STAT NOVEMBER 21, 2018)
"China introduces sweeping reforms to crack down on academic misconduct"
"Initiatives include a national list of cases, a blacklist of ‘poor quality’ journals and a government agency that is responsible for policing misconduct."
- "China is getting tough on scientific misconduct. The country’s most powerful bodies, the Chinese Communist Party and the State Council, introduced a raft of reforms on 30 May aimed at improving integrity across the research spectrum, from funding and job applications to peer-review and publications.
Under the new policy, the Ministry of Science and Technology (MOST) will be responsible for managing investigations and ruling on cases of scientific misconduct, a role previously performed by individual institutions. And for the first time, misconduct cases will be logged in a national database that is currently being designed by MOST.
Inclusion in the list could disqualify researchers from future funding or research positions, and might also affect their ability to get jobs outside academia. The Chinese Academy of Social Sciences will oversee the same process for social scientists." (Source: "China introduces sweeping reforms to crack down on academic misconduct" David Cyranoski. Nature VOL 558. 08 June 2018. p. 171. View/Download pdf here.)
Research Misconduct (continued)
The Norwegian National Research Ethics Committees
- "The National Committee for Research Ethics in Science and Technology (NENT) is part of the Norwegian National Research Ethics Committees, an independent administrative agency under the Ministry of Education and Research. NENT is an impartial advisory body providing guidance and advice on research ethics, and the guidelines are important tools for promoting good scientific practice in the Norwegian research system." (Source: Back cover of: "Guidelines for research ethics in science and technology" Issued by The Norwegian National Committee for Research Ethics in Science and Technology (2016), p. 10. Text: The Norwegian National Committees for Research Ethics, Last updated: Tuesday, June 28, 2016. ISBN 978-82-7682-075-1)
- Fraud and plagiarism
"It may be tempting to dress up in borrowed plumage (plagiarise/refrain from referring to one’s sources), cook data (falsify data) or simply invent (fabricate) data, and disregard the fact that this violates fundamental and internationally accepted rules for good research practice." (Source: "Fraud and plagiarism" Text: Torkild Vinther, Last updated: 25. February 2016. The Norwegian National Research Ethics Committees) - "Scientific integrity
Researchers are responsible for respecting the research results of others and for exercising good scientific practice. Researchers must not conceal, misrepresent or falsify anything, whether in the planning, execution or reporting of the research. Plagiarism involves presenting the ideas or research of others as one’s own.
The individual researcher has an independent responsibility not to accept departures from good scientific practice, on his or her own account or that of others. 2 Researchers who discover or are made aware of errors in their research, must admit the error, correct it, and ensure that the consequences of the error are minimal."
(Source: "Guidelines for research ethics in science and technology" Issued by The Norwegian National Committee for Research Ethics in Science and Technology (2016), p. 10. Text: The Norwegian National Committees for Research Ethics, Last updated: Tuesday, June 28, 2016. ISBN 978-82-7682-075-1) - "Good citation practice
It is in the nature of research to build on research by others. Researchers who take advantage of the ideas and research by others, both published and unpublished, must acknowledge this accurately, so that it is clear what the researcher’s own contribution is. Researchers must give a balanced and correct presentation of the research of others. Citations make research traceable and verifiable."
[2 The Act on ethics and integrity in research (the Research Ethics Act).] (Source: ibid.) - "5 Researchers must respect the contributions of other researchers and observe standards of authorship and cooperation.
Researchers must observe good publication practice. They must clarify individual responsibilities in group work as well as the rules for co-authorship. Honorary authorship is unacceptable. When several authors contribute, each authorship must be justified. Justified authorship is defined by four criteria, in accordance with the criteria drawn up by the International Committee of Medical Journal Editors (ICMJE) 3 :
a) Researchers must have made a substantial contribution to the conception and design or the data acquisition or the data analysis and interpretation; and
b) researchers must have contributed to drafting the manuscript or critical revision of the intellectual content of the publication; and
c) researchers must have approved the final version before publication; and
d) researchers must be able to accept responsibility for and be accountable for the work as a whole (albeit not necessarily all technical details) unless otherwise specified.
All authors in a multidisciplinary publication must be able to account for the part or parts for which they have been responsible in the research work, and which part or parts are the responsibility of other contributors.
All those who meet criterion a) must be able to meet b) and c). Contributors who do not fulfil all the criteria must be acknowledged.
Research Misconduct (continued)
Research Integrity in Norway (RINO) – a study of scientific misconduct and questionable research practices
- Project to investigate research integrity among all researchers in Norway
"The project, called RINO – Research Integrity in Norway, is a collaboration between UiB, The Norwegian National Research Ethics Committees (FEK) and The Western Norway University of Applied Sciences (HVL). Helene Ingierd, one of FEKs representatives to the RINO-project, says it can provide important knowledge."
"The National Research Ethics Committees (FEK) is an advisory body in the field of research ethics, and we are also supposed to spread knowledge. In order to exert this role in the best possible way, we need a knowledge base", said Ingierd." (Source: The Norwegian National Research Ethics Committee website: Project to investigate research integrity among all researchers in Norway. Text: Ingrid S. Torp, Last updated: 12. February 2018. Also see RINO Project PDF:Research Integrity in Norway (RINO) – a study of scientific misconduct and questionable research practices)
- The RINO Project is featured on the website of the European Network of Research Integrity Offices (ENRIO). Norway is a founding member of ENRIO. Torkild Vinther, Director of The National Commission for the Investigation of Research Misconduct, is Vice-Chair of ENRIO. Moreover, Torkild Vinther is a member of the Reference Group for the RINO Project. (Source: ENRIO website: http://www.enrio.eu/news-activities/research-integrity-norway-rino/, RINO Project website: https://www.uib.no/en/rino and RINO Project First Interim Report PDF)
- Torkild Vinther authored a book chapter titled "SKJØNNSUTØVELSE I STADFESTING AV PLAGIAT" ("EXERCISE DISCRETION IN CONFIRMATION OF PLAGIARISM") in: Etisk skjønn i forskning (Ethical discretion in research). Included below is an excerpt form this article in which Torkild Vinther makes the important point that along with text plagiarism, failure to credit the source of one's ideas is also plagiarism.
- "Although the main focus is text plagiarism, it is still necessary to mention explicitly where you have acquired your ideas (models, concepts, hypotheses, explanations, metaphors, operationalizations, conclusions, etc.). Cosmetic or minor changes do not remove the requirement to credit the source. If you do not, it will appear that the idea is one's own."
"Selv om hovedfokus er tekstplagiat, skal det likevel nevnes at man eksplisitt må vise til hvor man har hentet sine ideer (modeller, begreper, hypoteser, forklaringer, metaforer, operasjonaliseringer, konklusjoner osv.). Kosmetiske eller mindre endringer fjerner ikke kravet om å kreditere kilden. Gjør man ikke det, vil det fremstå som om ideen er ens egen." (Source: "SKJØNNSUTØVELSE I STADFESTING AV PLAGIAT" in Etisk skjønn i forskning edited by Hallvard J. Fossheim og Helene Ingierd 2015 (Redaksjonelt arbeid, utvalg og introduksjon (Editorial work, selection and introduction)) p. 87-98) [Note: About the editors: Hallvard J. Fossheim is Professor of Philosophy, University of Bergen and the (2010-2014) secretariat leader of The National Research Ethical Committee for Social Sciences and Humanities (NESH) and the National Committee for the Evaluation of Human Resource Research (Skelettutvalget); and, Helene Ingierd is Director, The National Committee for Research Ethics in Science and Technology (NESH)]
- "Although the main focus is text plagiarism, it is still necessary to mention explicitly where you have acquired your ideas (models, concepts, hypotheses, explanations, metaphors, operationalizations, conclusions, etc.). Cosmetic or minor changes do not remove the requirement to credit the source. If you do not, it will appear that the idea is one's own."
- The RINO Project’s first interim report (presented June 2018) showed the percentages of the RINO survey respondents who know colleagues who have participated in questionable or unacceptable practices of Fabrication, Falsification & Plagiarism over the last 3 years to be 2.2%, 2.2% & 13.8%, respectively (Table 7, p. 16/English; 13/Norwegian). It would appear Plagiarism is more than 6 times more prevalent than Fabrication and Falsification.
- "The RINO survey shows that, of the three mentioned practices, knowledge of colleagues’ plagiarism is far more widespread than knowledge of colleagues fabricating or falsifying data."
"Almost 14%, or around 1 in 7 respondents, state that they are aware of colleagues plagiarising others’ work in the last three years.The corresponding figure for fabricating or falsifying data is just 2%, or 1 in 50." (Source: English: "Research integrity in Norway – results from a nationwide survey on research ethics" Johs. Hjellbrekke, Laura Drivdal, Helene Ingierd, Ole Bjørn Rekdal, Heidi Skramstad, Ingrid S. Torp og Matthias Kaiser. 2018. p. 16. ISBN: 978-82-7682-082-9) [Note: RINO Project survey questionnaire; RINO website: RESEARCH INTEGRITY IN NORWAY (RINO)]
"RINO-undersøkelsen viser at av de tre nevnte praksisene er kjennskap til kollegaers plagiering langt mer utbredt enn kjennskap til kollegaers datafabrikkering eller dataforfalskning."
"Nesten 14 %, eller drøyt 1 av 7 respondenter, oppgir at de kjenner til kollegaers plagiering av andres arbeid de siste tre årene. For fabrikkering eller forfalsking av data er tilsvarende tall kun 2 %, eller 1 av 50." (Source: "
- "The RINO survey shows that, of the three mentioned practices, knowledge of colleagues’ plagiarism is far more widespread than knowledge of colleagues fabricating or falsifying data."
|
Figure: Slippery slope between honest errors and intentional fraud, with examples in the middle
Horizontal axis represents extent of deviation from acceptable scientific behavior. Vertical axis represents extent of blame, from excusable errors, via non-intentional but still blamable deviance, to wilful actions. |
Research Misconduct (continued)
"New Classification of Research Misconduct from the Viewpoint of Truth, Trust, and Risk"
Research Misconduct (continued)
"Nine pitfalls of research misconduct"
"Academic leaders must audit departments for flaws and strengths, then tailor practices to build good behaviour, say C. K. Gunsalus and Aaron D. Robinson."
"C.K.G. coined the mnemonic TRAGEDIES (Temptation, Rationalization, Ambition, Group and authority pressure, Entitlement, Deception, Incrementalism, Embarrassment and Stupid systems) to capture the interlocking factors that can lead scientists astray 2 (see ‘A table of tragedies’).
A Table of Tragedies
The factors that lead to bad decisions can be represented by the mnemonic TRAGEDIES. Here are some examples of each pitfall. Recognizing these and responding appropriately can save a career and strengthen science.
"New Classification of Research Misconduct from the Viewpoint of Truth, Trust, and Risk"
- "ABSTRACT
Fabrication, Falsification and Plagiarism (FFP) and Questionable Research Practice (QRP) have been used worldwide in the classification of research misconduct. However, FFP comprises two distinct categories of misconduct: FF is extreme research misconduct that betrays truth, while P undermines trust of science community. Irreproducibility and inadequate practice of research also betray trust. Research misconduct has the potential to cause serious risk of safety in daily life. The proposed classification system is outlined as follows: Class I misconduct: Betrayal of the truth: (1) Fabrication and (2) Falsification. Class II misconduct: Betrayal of trust: (1) Plagiarism of text ; [(2)] Irreproducibility; and (3) Inadequate research practice. Class III misconduct: Risk to safety of health and industrial products: (1) Risk to safety of health and (2) Risk to safety of industrial products. The proposed classification reflects deeper values of truth, trust, and risk more directly than the previous classification and elucidates issues about nature and significance of misconduct." (Source: "New Classification of Research Misconduct from the Viewpoint of Truth, Trust, and Risk" Commentary. Toshio Kuroki , M.D., Ph.D. Accountability in Research Policies and Quality Assurance Volume 25, 2018 - Issue 7-8. Pages 404-408 | Accepted author version posted online: 14 Nov 2018, Published online: 14 Nov 2018)
- "Abstract
The issue of how to best minimize scientific misconduct remains a controversial topic among bioethicists, professors, policymakers, and attorneys. This paper suggests that harsher criminal sanctions against misconduct, better protections for whistleblowers, and the creation of due process standards for misconduct investigations are urgently needed. Although the causes of misconduct and estimates of problem remain varied, the literature suggests that scientific misconduct-fraud, fabrication, and plagiarism of scientific research-continues to damage public health and trust in science. Providing stricter criminal statutes against misconduct is necessary to motivate whistleblowers and deter wrongdoers, and the provision of basic due process protections is necessary for ensuring a fair and balanced misconduct investigation." (Source: "Using criminalization and due process to reduce scientific misconduct." Sovacool BK. Am J Bioeth. 2005 Sep-Oct;5(5):W1-7. PMID: 16179287 DOI: 10.1080/15265160500313242)
Research Misconduct (continued)
"Nine pitfalls of research misconduct"
"Academic leaders must audit departments for flaws and strengths, then tailor practices to build good behaviour, say C. K. Gunsalus and Aaron D. Robinson."
"C.K.G. coined the mnemonic TRAGEDIES (Temptation, Rationalization, Ambition, Group and authority pressure, Entitlement, Deception, Incrementalism, Embarrassment and Stupid systems) to capture the interlocking factors that can lead scientists astray 2 (see ‘A table of tragedies’).
A Table of Tragedies
The factors that lead to bad decisions can be represented by the mnemonic TRAGEDIES. Here are some examples of each pitfall. Recognizing these and responding appropriately can save a career and strengthen science.
- Temptation “Getting my name on this article would look really good on my CV.”
- Rationalization “It’s only a few data points, and those runs were flawed anyway.”
- Ambition “The better the story we can tell, the better a journal we can go for.”
- Group and authority pressure “The PI’s instructions don’t exactly match the protocol approved by the ethics review board, but she is the senior researcher.”
- Entitlement “I’ve worked so hard on this, and I know this works, and I need to get this publication.”
- Deception “I’m sure it would have turned out this way (if I had done it).”
- Incrementalism “It’s only a single data point I’m excluding, and just this once.”
- Embarrassment “I don’t want to look foolish for not knowing how to do this.”
- Stupid systems “It counts more if we divide this manuscript into three submissions instead of just one.”"
|
“There’s not one magic bullet, it’s being aware of — and taking responsibility for — our professional conduct and our professional environments.”
|
(Source: "Nine pitfalls of research misconduct" C. K. Gunsalus & Aaron D. Robinson. Nature 557, 297-299 (2018). 16 May 2018 doi: 10.1038/d41586-018-05145-6) [Note: C. K. Gunsalus is the director of the National Center for Professional and Research Ethics (NCPRE) at the University of Illinois Urbana-Champaign. Also, C. K. Gunsalus heads C. K. Gunsalus and Associates Consulting)
"Misconduct Expert Dissects Duke Scandal"
"Penalty Too Light"
"Misconduct Expert Dissects Duke Scandal"
- "By now, in our post-Watergate society, we should all understand that even the appearance of a cover-up is enough to provoke interest. While the lessons of painful experience do not come intuitively to investigators whose work is challenged, institutional officials should understand—and be able to convey persuasively—the reality that the moment at which credible questions surface about the validity of work is the precise time to seek an outside perspective, not to circle the wagons."
(...)
"Final Thoughts
Academic research operates on trust. It is critical to be able to trust what’s in the published literature. As the Nobel Laureate Joshua Lederberg put it, “above all, a publication is inscription under oath, a testimony.” (10) The authors on the byline are directly responsible to the readers. Potti had a great number of co-authors. How is it that none of them saw the problems that were obvious to Baggerly, Coombes, and Perez?
Ironically, because the Institute of Medicine review committee was informed that Duke had contacted all 162 collaborators who were co-authors on all 40 papers by Anil Potti, and “in no instance did anyone make any inquiries or call for retractions until contacted by Duke,” the IOM committee concluded that “This experience suggests the need for co-authors to have more shared responsibility for the integrity of the published research.” (p. 251)
Too bad they didn’t hear about the one person who saw such significant problems with the work that he asked for his name to be withdrawn: the medical student who took his authorship obligations so seriously that he set his own career back in order to honor them. (Source: "Misconduct Expert Dissects Duke Scandal" By C.K. Gunsalus. The Cancer Letter Jan. 23, 2015)
"Penalty Too Light"
- "What does it say about our national commitment to research integrity that the Department of Health and Human Services’ Office of Research Integrity has concluded that a five-year ban on federal research funding for one individual researcher is a sufficient response to a case involving millions of taxpayer dollars, completely fabricated data, and hundreds to thousands of patients in invasive clinical trials?
This week, ORI released a notice of “final action” in the case of Anil Potti, M.D. The ORI found that Dr. Potti engaged in several instances of research misconduct and banned him from receiving federal funding for five years.
The principles involved are important and the facts complicated. This was not just a matter of research integrity. This was also a case involving direct patient care and millions of dollars in federal and other funding. The duration and extent of deception were extreme. The case catalyzed an Institute of Medicine review of genomics in clinical trials and attracted national media attention.
If there are no further conclusions coming from ORI and if there are no other investigations under way—despite the importance of the issues involved and the five years that have elapsed since research misconduct investigation began, we do not know--a strong argument can be made that neither justice nor the research community have been served by this outcome." (Source: "Penalty Too Light" A Guest Editorial by Keith Baggerly and C.K. Gunsalus. The Cancer Letter Nov. 13, 2015)
(Source: U.S. Department of Health and Human Services (HHS) Office of Research Integrity (ORI) "Introduction to the Responsible Conduct of Research, Revised Edition August 2007" Nicholas H. Steneck, illustrations by David Zinn. ISBN 978-0-16-072285-1)
Retractions
COPE: "Retractions: Guidance from the Committee on Publication Ethics (COPE)"
COPE: "Retraction Guidelines - Summary"
Editors should consider retracting a publication [1] if:
Retraction Watch
"Tracking retractions as a window into the scientific process"
COPE: "Retractions: Guidance from the Committee on Publication Ethics (COPE)"
- "The Purpose of Retraction
Retraction is a mechanism for correcting the literature and alerting readers to publications that contain such seriously flawed or erroneous data that their findings and conclusions cannot be relied upon. Unreliable data may result from honest error or from research misconduct.
Retractions are also used to alert readers to cases of redundant publication (ie, when authors present the same data in several publications), plagiarism, and failure to disclose a major competing interest likely to influence interpretations or recommendations.
The main purpose of retractions is to correct the literature and ensure its integrity rather than to punish authors who misbehave." (Source: "Retractions: Guidance from the Committee on Publication Ethics (COPE)" Elizabeth Wager, Virginia Barbour, Steven Yentis, Sabine Kleinert, and on behalf of COPE Council. Croat Med J. 2009 Dec; 50(6): 532–535. p. 533-533. doi: [10.3325/cmj.2009.50.532])
COPE: "Retraction Guidelines - Summary"
Editors should consider retracting a publication [1] if:
- They have clear evidence that the findings are unreliable, either as a result of major error (eg, miscalculation or experimental error), or as a result of fabrication (eg, of data) or falsification (eg, image manipulation)
- It constitutes plagiarism
- The findings have previously been published elsewhere without proper attribution to previous sources or disclosure to the editor, permission to republish, or justification (ie, cases of redundant publication)
- It contains material or data without authorisation for use
- Copyright has been infringed or there is some other serious legal issue (eg, libel, privacy)
- It reports unethical research
- It has been published solely on the basis of a compromised or manipulated peer review process
- The author(s) failed to disclose a major competing interest (a.k.a. conflict of interest) that, in the view of the editor, would have unduly affected interpretations of the work or recommendations by editors and peer reviewers.
- Be linked to the retracted article wherever possible (ie, in all online versions)
- Clearly identify the retracted article (eg, by including the title and authors in the retraction heading or citing the retracted article)
- Be clearly identified as a retraction (ie, distinct from other types of correction or comment)
- Be published promptly to minimise harmful effects • Be freely available to all readers (ie, not behind access barriers or available only to subscribers)
- State who is retracting the article
- State the reason(s) for retraction
- Be objective, factual and avoid inflammatory language.
[1] These guidelines are intended to apply primarily to journal articles but may be applicable to book chapters, abstracts, preprints and other published documents.
(Source: Version 2: COPE Council. "COPE Guidelines: Retraction Guidelines" November 2019; Version 1: Published September 2009. DOI: https://doi.org/10.24318/cope.2019.1.4 URL: https://publicationethics.org/retraction-guidelines PDF: https://publicationethics.org/files/retraction-guidelines.pdf)
Retraction Watch
"Tracking retractions as a window into the scientific process"
- "The Database of 18,000 Retracted Scientific Papers Now Online
(...)
All of that could change, though, because the largest database of scientific retractions just went live and makes the process a whole lot easier. Retraction Watch Database is designed expressly for finding out whether any given study is still legit. The next time you read an article or hear someone say, "studies show that talking is bad for you," you can head over to the site and see what's what.
The database is an offshoot of a blog started in 2010 by two medical reporters, Ivan Oransky and Adam Marcus. One of the highlights of the blog is a list of the 10 studies most often cited even though they've been retracted. The notorious and long-since debunked study linking autism to vaccinations is there, as well as a 2013 paper called "Primary Prevention of Cardiovascular Disease with a Mediterranean Diet" (sorry foodies!).
If you question the need for such a database, consider this: Some studies, such as one claiming to have discovered a protein that mimics insulin, have actually been cited more often after they were retracted than before." (Source: "Database of 18,000 Retracted Scientific Papers Now Online" in HowStuffWorks.com BY OISIN CURRAN NOV 6, 2018) [Note: Also see: "Retraction Watch Database User Guide"]
- "Nearly a decade ago, headlines highlighted a disturbing trend in science: The number of articles retracted by journals had increased 10-fold during the previous 10 years. Fraud accounted for some 60% of those retractions; one offender, anesthesiologist Joachim Boldt, had racked up almost 90 retractions after investigators concluded he had fabricated data and committed other ethical violations. Boldt may have even harmed patients by encouraging the adoption of an unproven surgical treatment. Science, it seemed, faced a mushrooming crisis." (Source: "What a massive database of retracted papers reveals about science publishing’s ‘death penalty’" By Jeffrey Brainard, Jia You. Science Oct. 25, 2018, 2:00 PM)
"The burden of misconduct
The majority of retractions have involved scientific fraud (fabrication, falsification, and plagiarism) or other kinds of misconduct (such as fake peer review)."
|
"Changing infractions
The proportion of retractions involving plagiarism of text —stealing someone else's or duplicating one's own— has risen; one cause appears to be the introduction in 2004 of iTehnticate, an internet-based plagiarism detection service. Fake peer reviews occur when authors give journals email addresses that they control, allowing them to review their own manuscripts. Flawed images include instances of intentional manipulation and of error." |
|
Source: "What a massive database of retracted papers reveals about science publishing’s ‘death penalty’" Jeffrey Brainard, Jia You. Science Oct. 25, 2018, 2:00 PM)
|
"What’s Behind Big Science Frauds?"
"A Sharp Rise in Retractions Prompts Calls for Reform"
"Misconduct accounts for the majority of retracted scientific publications"
"Why you can’t trust science"
"Rector at Oslo and Akershus University College, Curt Rice, explains why people have good reasons to doubt research, and what needs to be done to improve the situation."
("Rektor på Høgskolen i Oslo og Akershus, Curt Rice, forklarer her hvorfor folk har gode grunner til å tvile på forskning, og hva som må gjøres for å bedre situasjonen.")
Securing research integrity in the publishing process - Tamara Welschot, Ombudssymposium 2018
- "Retractions can be good things, since even scientists often fail to acknowledge their mistakes, preferring instead to allow erroneous findings simply to wither away in the back alleys of unreproducible literature. But they don’t surprise those of us who are familiar with how science works; we’re surprised only that retractions aren’t even more frequent.Remember that study showing vaccines were linked to autism? The time scientists claimed to have cloned human embryonic stem cells? Or that simple, easy way that was supposed to revolutionize the creation of such stem cells? Those were all frauds published in the world’s top scientific journals — The Lancet, Science and Nature. The vaccine scare has been associated with a surge in cases of measles, some of them deadly.
(...)
Economists like to say there are no bad people, just bad incentives. The incentives to publish today are corrupting the scientific literature and the media that covers it. Until those incentives change, we’ll all get fooled again." (Source: "What’s Behind Big Science Frauds?" Opinion, OP-ED CONTRIBUTORS Adam Marcus and Ivan Oransky. The New York Times May 11, 2015. "A version of this article appears in print on May 23, 2015, on Page A19 of the New York edition with the headline: What’s Behind Big Science Frauds?.")
"A Sharp Rise in Retractions Prompts Calls for Reform"
- "Dr. Fang became curious how far the rot extended. To find out, he teamed up with a fellow editor at the journal, Dr. Arturo Casadevall of the Albert Einstein College of Medicine in New York. And before long they reached a troubling conclusion: not only that retractions were rising at an alarming rate, but that retractions were just a manifestation of a much more profound problem — “a symptom of a dysfunctional scientific climate,” as Dr. Fang put it.
Dr. Casadevall, now editor in chief of the journal mBio, said he feared that science had turned into a winner-take-all game with perverse incentives that lead scientists to cut corners and, in some cases, commit acts of misconduct.
“This is a tremendous threat,” he said.
Last month, in a pair of editorials in Infection and Immunity, the two editors issued a plea for fundamental reforms. They also presented their concerns at the March 27 meeting of the National Academies of Sciences committee on science, technology and the law.
(...)
Dr. Ness likens scientists today to small-business owners, rather than people trying to satisfy their curiosity about how the world works. “You’re marketing and selling to other scientists,” she said. “To the degree you can market and sell your products better, you’re creating the revenue stream to fund your enterprise.” (Source: "A Sharp Rise in Retractions Prompts Calls for Reform" By CARL ZIMMER The New York TImes APRIL 16, 2012. "A version of this article appears in print on April 17, 2012, on Page D1 of the New York edition with the headline: A Sharp Rise in Retractions Prompts Calls for Reform.")
"Misconduct accounts for the majority of retracted scientific publications"
- "Abstract
A detailed review of all 2,047 biomedical and life-science research articles indexed by PubMed as retracted on May 3, 2012 revealed that only 21.3% of retractions were attributable to error. In contrast, 67.4% of retractions were attributable to misconduct, including fraud or suspected fraud (43.4%), duplicate publication (14.2%), and plagiarism (9.8%). Incomplete, uninformative or misleading retraction announcements have led to a previous underestimation of the role of fraud in the ongoing retraction epidemic. The percentage of scientific articles retracted because of fraud has increased ∼10-fold since 1975. Retractions exhibit distinctive temporal and geographic patterns that may reveal underlying causes." (Source: "Misconduct accounts for the majority of retracted scientific publications"
Ferric C. Fang, R. Grant Steen, and Arturo Casadevall. PNAS October 16, 2012 109 (42) 17028-17033; https://doi.org/10.1073/pnas.1212247109. Edited by Thomas Shenk, Princeton University, Princeton, NJ, and approved September 6, 2012 (received for review July 18, 2012)) - "The rise in the rate of retractions raises concern about the health of the scientific enterprise itself (32). Although articles retracted because of fraud represent a very small percentage of the scientific literature (Fig. 1B), it is important to recognize that: i) only a fraction of fraudulent articles are retracted; (ii) there are other more common sources of unreliability in the literature (41⇓⇓–44); iii) misconduct risks damaging the credibility of science; and (iv) fraud may be a sign of underlying counterproductive incentives that influence scientists (45, 46). A better understanding of retracted publications can inform efforts to reduce misconduct and error in science." (Source: Ibid.)
"Why you can’t trust science"
"Rector at Oslo and Akershus University College, Curt Rice, explains why people have good reasons to doubt research, and what needs to be done to improve the situation."
("Rektor på Høgskolen i Oslo og Akershus, Curt Rice, forklarer her hvorfor folk har gode grunner til å tvile på forskning, og hva som må gjøres for å bedre situasjonen.")
- "A crisis for science?
I believe the problems discussed here are a crisis for science and the institutions that fund and carry out research. We have a system for communicating results in which the need for retraction is exploding, the replicability of research is diminishing, and the most standard measure of journal quality is becoming a farce. Indeed, the ranking of journals by impact factor is at the heart of all three of these problems. Brembs and Munafò conclude that the system is so broken it should be abandoned.
Getting past this crisis will require both systemic and cultural changes. Citations of individual articles can be a good indicator of quality, but the excellence of individual articles does not correlate with the impact factor of the journals in which they are published. When we have convinced ourselves of that, we must see the consequences it has for the evaluation processes essential to the construction of careers in science and we must push nascent alternatives such as Google Scholar and others forward.
Politicians have a legitimate need to impose accountability, and while the ease of counting – something, anything – makes it tempting for them to infer quality from quantity, it doesn’t take much reflection to realize that this is a stillborn strategy. As long as we believe that research represents one of the few true hopes for moving society forward, then we have to face this crisis.
It will be challenging, but there is no other choice." (Source: "Why you can’t trust science" Curt Rice, Rektor, Høgskolen i Oslo og Akershus. Khrono. PUBLISERT MANDAG 25. SEPTEMBER 2017 - 19:42 SIST OPPDATERT ONSDAG 27. SEPTEMBER 2017 - 16:58)
Securing research integrity in the publishing process - Tamara Welschot, Ombudssymposium 2018
- Tamara Welschot
"Tamara Welschot is since May 2017 Head of Advisory and Assurance, Research Integrity Springer Nature. Tamara looks after resources on research integrity and publication ethics for Editors, Authors and Reviewers and oversees projects on preventing and detecting misconduct. Prior to that Tamara was Director of Research Integrity and Publishing Services with overall responsibility for Springer’s strategy and standards on publication ethics, providing expert advice on ethical issues, and overseeing other activities such as annual year reporting for Journal Editors, abstracting & indexing and digital preservation." (Source: Tamara Welschot - International Association of STM Publishers) [Also see: "Springer Nature's reply on fake review" Tamara Welschot. Nature volume 546, page 210. 08 June 2017]
"Legal threats, opacity, and deceptive research practices: A look at more than 100 retractions in business and management"
"Research misconduct in business and management studies: causes, consequences and possible remedies" (original)
"The visibility of scientific misconduct: A review of the literature on retracted journal articles"
"The ethics of scholarly publishing: exploring differences in plagiarism and duplicate publication across nations"
Retractions (continued)
"Status of retraction notices for biomedical publications associated with research misconduct"
"Retracted papers die hard: Diederik Stapel and the enduring influence of flawed science"
- "RW: You write that “Classifying these reasons was not straightforward,” and you quote an earlier paper as saying that “For masterful obfuscation, it’s hard to beat the wording of some retractions.” Can you explain?
DT: Many retraction statements just baldly announce that a paper has been retracted without giving any explanation. Examples: ‘The author has retracted this paper.’ ‘This article has been retracted.’ Others simply say things like ‘The retraction has been agreed before print publication based on discussions about the presentation of the empirical results.’ Such statements tell us very little. It may be that editors are keen to avoid giving offence, and are also seeking to avoid the possibility of legal action. Nevertheless, we don’t believe that vagueness is defensible. It makes it difficult to ascertain the extent of various problems such as fraud and plagiarism. Where flawed analysis is the issue, a refusal to spell it out in detail prevents other researchers learning from whatever mistakes have been made. They are therefore more likely to be repeated. Clear statements of the reasons for retractions are vital. And it is more important than this in disciplines where faulty research has implications for human well-being and may well have life threatening consequences." (Source: "Legal threats, opacity, and deceptive research practices: A look at more than 100 retractions in business and management" Ivan Oransky Retraction Watch November 23, 2018) [Note: RW = Retraction Watch; DT = Dennis Tourish, author of "Research misconduct in business and management studies: causes, consequences and possible remedies"] - "RW: You found that “Twelve papers in our database were retracted with no clear reason provided,” and one of your recommendations is to “Require journals to make clearer statements of reasons for retraction.” We have been calling for this since we launched in 2010, and others have done the same. Are you hopeful that things will change?
DT: In the short term, no. I don’t feel confident about this. There still seems to be a great deal of complacency about the level of misconduct, and too many editors, publishers and academics continue to ignore serious problems. Some editors and journals appear to be playing lip service to the COPE Guidelines. The journal webpages say the journal complies with them, but one of these is that clear reasons be given for retractions. Indicating who requested the retraction is not the same thing as giving a reason for it. In the longer term, a failure to give clear reasons for retraction is so manifestly absurd that pressure to change can only grow. However, it would be good if publishers and journals acted before they are compelled to!" (Source: ibid.)
"Research misconduct in business and management studies: causes, consequences and possible remedies" (original)
- "Abstract
...Our aim is to promote debate about the causes and consequences of research misconduct and to suggest possible remedies. Drawing on corruption theory, we suggest that a range of institutional, environmental and behavioural factors interact to provide incentives that sustain research misconduct. We explore the research practices that have prompted retractions. We contend that some widely-used, but questionable research practices, should be challenged so as to promote stronger commitment to research integrity and to deter misconduct. We propose eleven recommendations for action by authors, editors, publishers and the broader scientific community. " - "RECOMMENDATIONS
Flowing from the analysis above, we make eleven recommendations that are directed to authors, journal editors and publishing houses, and the broader academic community. These recommendations seek to address the environmental, institutional and behavioural factors that are complicit in nurturing research fraud and questionable research practices." - "Recommendations to Authors
1. Maintain proper data collection records
2. Clarify responsibility for data collection
3. Specify all statistical analyses that were conducted - Recommendations to Journal Editors and Publishing Houses
4. Require journals to make clearer statements of reasons for retraction
5. Investigate the whole body of work of authors who have papers retracted for fraud
6. Develop clearer rules for disclosure of penalties and conflicts of interest
7. Label all retracted papers as ‘retracted’ - Recommendations to the Broader Academic Community
8. Redefine p-hacking as a deceptive research practice
9. Substitute the term ‘deceptive research practice’ for ‘questionable research practices’
10. Develop research misconduct policies
11. Strengthen the education of PhD students to include more explicit, detailed and frequent consideration of poor research practices and the need for ethical research."
(Source: Tourish, Dennis and Craig, Russell (2018) Research misconduct in business and management studies: causes, consequences and possible remedies. Journal of Management Inquiry. ISSN 1056-4926. PDF - Accepted Version Download (471kB))
"The visibility of scientific misconduct: A review of the literature on retracted journal articles"
- "Abstract (...) This study treats retractions as an emerging institution that renders scientific misconduct visible, thus, following up on the sociology of deviance and its focus on visibility. The article shows that retractions, by highlighting individual cases of misconduct and general policies for preventing misconduct while obscuring the actors and processes through which retractions are effected, produce highly fragmented patterns of visibility. These patterns resemble the bifurcation in current justice systems." (Source: "The visibility of scientific misconduct: A review of the literature on retracted journal articles" Felicitas Hesselmann, Verena Graf, Marion Schmidt, Martin Reinhart. Current Sociology Review 2017, Vol. 65(6) 814–845)
- "Every retraction tells a story. At least half the time, that story involves misconduct or fraud. Consider Diederik Stapel, a Dutch psychologist who crafted his splashy findings about human behavior out of whole cloth; and Andrew Wakefield, whose spurious claims about the risks of childhood vaccinations and autism helped foment disastrous anti-vaccine campaigns. [Note: As reported by Martin Enserink ("Final Report on Stapel Also Blames Field As a Whole" Science 07 Dec 2012: Vol. 338, Issue 6112, pp. 1270-1271), the key message presented in the joint (Universities of Amsterdam, Groningen and Tilburg) final report for the Diederik Stapel case of scientific fraud "...is not just about Stapel but colleagues, co-authors, reviewers, and editors at even the most prestigious journals." See: "Flawed science: The fraudulent research practices of social psychologist Diederik Stapel" Levelt Committee, Noort Committee, Drenth Committee. Willem J.M. Levelt, Chair of the Coordinating Committee. 28 November 2012.] [Note: Also see: "The case of Diederik Stapel" Allegations of scientific fraud by prominent Dutch social psychologist are investigated by multiple universities. Mieke Verfaellie and Jenna McGwin. Psychological Science Agenda, the e-newsletter of the Science Directorate of the American Psychological Association, December 2011]
(...)
"While 18,000 retractions may sound like a lot, that amount is clearly just a fraction of the total number of papers that should be banished from the literature. We know that because some 2 percent of researchers admit, in anonymous surveys, to acts that are considered misconduct. And for every whistleblower who sees his or her work lead to a retraction, we hear from several who are met with silence or retaliation. The work is just beginning." (Source: "More science than you think is retracted. Even more should be." By Adam Marcus and Ivan Oransky. The Washington Post December 26 at 6:01 PM. "Adam Marcus, the managing editor of Gastroenterology & Endoscopy News, and Ivan Oransky, distinguished writer in residence at New York University’s Arthur Carter Journalism Institute and vice president for editorial at Medscape, are co-founders of Retraction Watch.")
"The ethics of scholarly publishing: exploring differences in plagiarism and duplicate publication across nations"
- "Introduction (...) The integrity of the published literature is of particular importance to librarians and other information professionals who endeavor to meet the information needs of health care providers, educators, researchers, patients, the public, and others. Librarians' success in fulfilling these needs depends on the available literature, and having reliable and accurate information is critical to research efforts, including systematic reviews; evidence-based practice; and decision making that can impact patient care and safety, the education of future health professionals, and the public's health." (Source: "The ethics of scholarly publishing: exploring differences in plagiarism and duplicate publication across nations" Kathleen A. Amos J Med Libr Assoc. 2014 Apr; 102(2): 87–91. doi: [10.3163/1536-5050.102.2.005], View/Dowload pdf here.)
- "Discussion (...) Perhaps the most striking illustration of unethical publishing practices can be found by considering plagiarism and duplicate publication together. Although distinct, these practices stem from a similar root in that both are issues of originality. Whether authors duplicate someone else's work or their own, they are not contributing new material to the knowledgebase in the field. Combining rates of plagiarism and duplicate publication highlights even more explicitly the originality problems occurring in select countries during this time period. Approximately 35% of the literature in the sample was retracted for plagiarism or duplicate publication, but more than 70% of the retracted literature by authors from Turkey, Italy, and Tunisia was duplicative in some way. Iran and India also had rates over 50%, and close to 50% of the retracted literature by Chinese authors was unoriginal. Several of these countries were also among those identified by Stretton et al. as having higher percentages of plagiarism retractions, using a definition that included self-plagiarism or duplicate publication 24. The United States, while not free of originality issues, had a combined rate of plagiarism and duplicate publication that was only slightly over 20%." (Source: ibid.)
Retractions (continued)
"Status of retraction notices for biomedical publications associated with research misconduct"
- "Abstract
In order to assess the status of retraction notices for publications involving research misconduct, we collected and analyzed information from the Office of Research Integrity website. This site lists confirmed instances of misconduct in research supported by the National Institutes of Health. Over a 10-year period, 200 publications derived from misconduct were identified. For 20.5% of those papers, no retraction notice was published. We found that the majority of these cases were from investigations concluded at least two years before our analysis, and thus are unlikely to be explainable by timing considerations. These findings demonstrate that retraction notices for papers associated with misconduct are often not published and suggest that clear, adherent policies are needed in this circumstance to correct the scientific record." (Source: "Status of retraction notices for biomedical publications associated with research misconduct" Daniel Drimer-Batca, Jonathan M Iaccarino, Alan. Research Ethics. FineFirst Published January 2, 2019 https://doi.org/10.1177/1747016118820496)
"Retracted papers die hard: Diederik Stapel and the enduring influence of flawed science"
- "Abstract
Self-correction is a defining feature of science. However, science’s ability to correct itself is far from optimal as shown, for instance, by the persistent influence of papers that have been retracted due to faulty methods or research misconduct. In this study, we track citations to the retracted work of Diederik Stapel. These citations provide a powerful indicative of the enduring influence of flawed science, as the (admittedly fabricated) data reported in these retracted papers provide no evidence for or against any hypothesis and this case of fraud was widely known due to the extensive media coverage of the scandal. Our data show that Stapel’s papers are still cited in a favorable way within and without the psychological literature. To ameliorate this problem, we propose that papers should be screened during the review process to monitor citations to retracted papers. " (Source: "Retracted papers die hard: Diederik Stapel and the enduring influence of flawed science" Luis Morís Fernández Miguel Vadillo. PsyArXiv Preprints, p. 2. CREATED ON June 19, 2019; LAST EDITED June 25, 2019; SUPPLEMENTAL MATERIALS osf.io/cwp93/) - "Although it is undeniable that science makes progress over time, it is far from clear that it does so in an optimal way. Questionable research practices and selective publication, coupled with a perverse structure of incentives in the scientific career, pave the way for the publication of ‘false discoveries’ that can remain unchallenged and influential for long periods of time (Ioannidis, 2012)."
(...)
"Unfortunately, retractions seem to be ineffective, as retracted papers are still cited in journals years after the retraction is made public (Bar-Ilan & Halevi, 2017; Cooper, 1992; Cosentino & Veríssimo, 2016). (Ibid., p. 3) - "Conclusions
Our results show that Stapel’s papers still receive an important number of positive citations years after their formal retraction. This is not the first study to show that retracted papers and disconfirmed findings remain cited as valid sources of knowledge (Bar-Ilan & Halevi, 2017; Tatsioni, Bonitsis, & Ioannidis, 2007), but we think that citations to Stapel’s papers are particularly worrying and relevant. Few cases of scientific malpractice and data fabrication have received the media coverage given to Stapel. Yet this has not prevented scientists from citing his retracted papers, even in top-quality psychology journals. Everything suggests that publishing retraction notices is an insufficient measure, even more if we consider that a simple retraction notice is unlikely to reach researchers on different fields." (Ibid., p. 7)
Systematic Manipulation of the Publication Process
"COPE: Systematic Manipulation of the Publication Process"
"Definition of Systematic Manipulation of the Publication Process is where an individual or a group of individuals have repeatedly used dishonest or fraudulent practices to:
"New COPE guidelines on publication process manipulation: why they matter"
"COPE: Systematic Manipulation of the Publication Process"
"Definition of Systematic Manipulation of the Publication Process is where an individual or a group of individuals have repeatedly used dishonest or fraudulent practices to:
- prevent or inappropriately influence the independent assessment of a piece of scholarly work by an independent peer.
- inappropriately attribute authorship of a piece of scholarly work.
- publish fabricated or plagiarised research.
- Peer review manipulation. This type of manipulation can occur directly by manipulation or hacking of the submission system of the journal. It can also occur when authors are able to suggest peer reviewers and input contact email addresses for these peer reviewers on the submission system of the journal. The authors may suggest fabricated names or names of real experts, but the contact email addresses are falsified so that all correspondence with the suggested peer reviewers is directed back to the authors. The manipulators then submit positive peer review reports to ensure the manuscript is accepted for publication.
- Authorship for sale/papermills. Another possibility is initially inserting the name of an accomplished guest author, especially for single-blind and open review, and then replacing the name during revision or after editorial acceptance.
- Substitution of a manuscript. Sometimes a high-quality manuscript is initially submitted (to ensure it passes peer review) and then a similar, but poorer quality manuscript (the authors’ own manuscript) is substituted after editorial acceptance"
(Source: "Systematic Manipulation of the Publication Process" Version 1: November 2018 COPE. publicationethics.org Developed in collaboration with: Springer Nature.)
"New COPE guidelines on publication process manipulation: why they matter"
- "Publication process manipulation is an industry-wide problem. The full extent of it is yet unknown and many publishers will have little experience of how to identify and manage it. For individual journals and editors, it is extremely difficult to spot. Even if detected, the competitive environment of commercial publishing together with confidentiality issues naturally discourage individual publishers from disclosing their methods of investigation, findings, and measures taken to prevent manipulation of their systems. However, to effectively tackle publication manipulation, a coordinated and collaborative approach is needed. With this thinking in mind, members of the Springer Nature Research Integrity Group [8], in collaboration with the Committee on Publication Ethics (COPE) [9], ran a workshop on publication manipulation at a COPE Publisher’s seminar in October 2016. At the seminar, a group of publishers were brought together to share their experiences of publication manipulation practices. The ultimate aim of the workshop was to agree a standardized approach to investigating and resolving such issues." (Source: "New COPE guidelines on publication process manipulation: why they matter" Jigisha Patel. Research Integrity and Peer Review 2018 3:13 https://doi.org/10.1186/s41073-018-0059-x Received: 12 November 2018 Accepted: 15 November 2018 Published: 26 November 2018. View/Download pdf here.)
Systematic Reviews
- Norway
"Systematic reviews are a prerequisite for evidence informed decisions because they provide a comprehensive review of what is known on the particular topic in question. By systematically reviewing existing research findings, new insights can be generated, knowledge gaps identified, and directions for future research found. The methods for developing a systematic review are characterised by 1) a clearly stated objective and pre-defined eligibility criteria for studies, 2) the use of explicit, reproducible methods, 3) a systematic search to identify all studies that would meet the eligibility criteria, 4) an assessment of the validity of the findings of the included studies, 5) a systematic presentation and synthesis of the characteristics and findings of the included studies. Randomized controlled trials play a key role in systematic reviews of the effectiveness of interventions, but other types of effectiveness-studies may also be relevant to include. The Norwegian Knowledge Centre for the Health Services produces systematic reviews to support well informed health policy making, clinical practice guidelines-development, and to contribute to evidence-based practices. The methods are described in the handbook ”Slik oppsummerer vi forskning” [“This is how we summarise research”], which is available online. The Knowledge Centre is also engaged in the wider global effort to produce systematic reviews, by preparing Cochrane- and Campbell-reviews, as well as systematic reviews that are published through regular scientific journals." (Source: "Systematic reviews on the effect of interventions" ("Systematiske oversikter om effekt av tiltak"), Gro Jamtvedt, Avdeling for kunnskapsoppsummering, Nasjonalt kunnskapssenter for helsetjenesten. G. Nor J Epidemiol 2013; 23 (2): 119-124. p. 119. Also see: Norwegian Institute of Public Health (NIPH) (Folkehelseinstituttet): "Slik oppsummerer vi forskning")
Term (i.e., Term of Pregnancy)
- "Gestation in singleton pregnancies lasts an average of 40 weeks (280 days) from the first day of the last menstrual period [LMP or LMPD] to the estimated date of delivery [EDD]." (Source: The American College of Obstetricians and Gynecologists Committee on Obstetric Practice & Society for Maternal-Fetal Medicine: Committee Opinion: Definition of Term Pregnancy). Also, the World Health Organisation (WHO) defines pregnancy duration as 40 weeks. However, it is important to note multiple studies, including a seminal Swedish study, Bergsjø et al. 1990, which analyzed 427,581 singleton births from the Swedish Birth Registry, have concluded human gestation is closer to 282 days.
- "In cases of reliable menstrual dates, the average duration from LMP to vaginal birth was 282 days (median), 281 days (mean) and 283 days (mode), remaining constant over the years of study. One standard deviation of the mean was approximately 13 days, varying slightly with age and parity. Ten per cent of these women gave birth post term (past 294 days). The duration of cesarean section births became shorter over the years, in spite of little change in cesarean section frequency (9.5% in 1976-7 and 10.9% in 1979-80). Mothers aged 35 and over tended to give birth 2 days earlier than those below 35. Second and subsequent children of mothers below 35 had slightly shorter gestations than first-borns. Boys were born earlier than girls, on average. When LMP was unreliable, the distribution of gestational lengths was wide. We also noted a seasonal rhythmicity in average duration of pregnancy, with consistent shortening in the month of December." (Source: "Duration of human singleton pregnancy. A population-based study" Per Bergsjø, Daniel W. Denman, Howard J. Hoffman, Olav Meirik. Acta Obstet Gynecol Scand. Jan 1990;69(3):197-207.)
Talk the talk & walk the walk
- Meaning: Back up one's talk with action.
Origin: 'Walk the walk' is almost always said in combination with 'talk the talk', for example, "if you’re going to talk the talk, you’ve got to walk the walk", or "walk it like you talk it". This is a 20th century American alternative to various old sayings which epitomise the notion that 'talk is cheap', for example 'actions speak louder than words' and 'practice what you preach'. The context for the use of any of these expressions is in response to what is seen as empty boasting. People who are accused of such are said (in the USA) to 'talk a good game' or (in the UK) to be 'all mouth and no trousers'. (Source: The Phrase Finder)
Ultrasound: History within Obstetrics & Gynecology
- "A short History of the development of Ultrasound in Obstetrics and Gynecology"
[ Part 1 ] [ Part 2 ] [ Part 3 ] [ Site Index ]
Dr. Joseph Woo (Copyright 1998-2002 Joseph SK Woo MBBS, FRCOG. All Rights Reserved. - "A Short History of Sonography in Obstetrics and Gynaecology"
S. Campbell (Facts Views Vis Obgyn. 2013; 5(3): 213–229. Download/View pdf here.)
Whistleblower
- "What is a Whistleblower?
In common terms, whistleblowers are individuals, typically employees, who use free speech rights to expose abuses of power that betray the public trust. Under the Whistleblower Protection Act (WPA), the primary law that protects non-intelligence federal employees, they are defined as employees who disclose information, either internally (to managers, organizational hotlines, etc.) or externally (to lawmakers, regulators, the media, watchdog organizations, etc.), that they reasonably believe evidences:- a violation of law, rule or regulation;
- gross mismanagement;
- a gross waste of funds;
- abuse of power; or
- a substantial and specific danger to public health or safety." (Source: "Working with Whistleblowers: A Guide for Journalists" Government Accountability Project - Truth Be Told, 2017. p. 4.)
- "Cynicism, or lack of belief that challenging misconduct will make a difference, overwhelmingly is the primary reason why would-be whistleblowers remain silent observers. Fear of retaliation is second, but a distant second. " (Source: Ibid., p. 5)
- "The Experts' mandate
The whistleblowing committee defines whistleblowing as “reporting cases of blameworthy conditions in the workplace to someone who might have the direct or indirect authority to do something about it.”
This is close to the English definition of whistleblowing. It covers everything from smaller breaches of regulations to comprehensive cases of a criminal nature.
“Blameworthy means what is illegal, unethical, immoral, illegitimate – in other words, this covers both legal and ethical matters,” said Anne Cathrine Frøstrup, the leader of the whistleblowing committee, as she presented the report. She then explained the background of the committee’s work, and its mandate. Paragraph 100 of the Norwegian Constitution on the freedom of speech, approved by parliament in September 2004, strengthened freedom of speech. The committee proposes that whistleblowing regulations refer to paragraph 100, in order to highlight how important freedom of speech is for the working environment and for people’s opportunity to speak out." (Source: "Norwegian experts: Whistleblowers need more protection" Berit Kvam. Nordic Labour Journal. MAR 23, 2018.)
- Relevant References
- Norway's Working Environment Act (Arbeidsmiljøloven)
- "Notification - values and protection: The alert committee's report on notification in the workplace" ("Varsling – verdier og vern: Varslingsutvalgets utredning om varsling i arbeidslivet") Anne Cathrine Frøstrup, leder. Norges offentlige utredninger 2018:6. Utredning fra utvalg oppnevnt ved kongelig resolusjon 11. november 2016. Avgitt til Arbeids- og sosialdepartementet 15. mars 2018. ISSN 0333-2306, ISBN 978-82-583-1357-8.